Air Quality
Most people living in or near urban areas have experienced air pollution first hand. Perhaps you've felt your eyes sting or your throat tickle as you drive an urban freeway. Or you've noticed black dust on window sills or window screens and realized that you are breathing in that dust as well.
AIR POLLUTANTS
Air pollution is largely the result of human activity. An air pollutant is an unwanted substance injected into the atmosphere from the Earth's surface by either natural or human activities. Air pollutants come as aerosols, gases, and particulates. In earlier chapters we've met aerosols—small bits of matter in the
air, so small that they float freely with normal air movements—and gases. Particulates are larger, heavier
particles that sooner or later fall back to Earth.
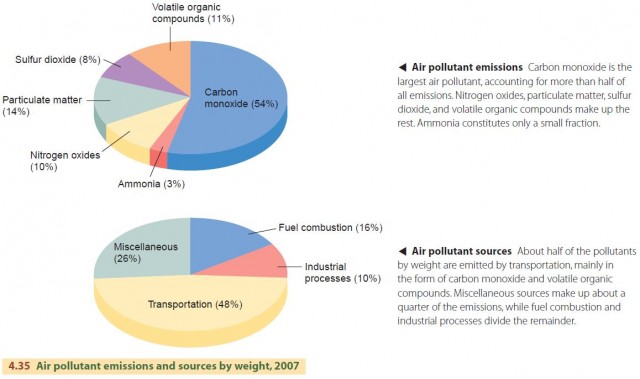
Most pollutants are generated by the everyday activities of large numbers of people, for example, driving cars, or through industrial activities, such as fossil fuel combustion or the smelting of mineral ores to produce metals. The most common air pollutant is carbon monoxide, followed by particulate matter, volatile organic compounds, nitrogen oxides, sulfur dioxide, and ammonia (Figure 4.35). Volatile organic compounds include evaporated gasoline, dry-cleaning fluids, and incompletely combusted fossil fuels. The buildup of these substances in the air can lead to many types of pollution, including acid deposition and smog.
SMOKE AND HAZE
When aerosols and gaseous pollutants are present in considerable density over an urban area, the resultant mixture is known as smog (Figure 4.36). Typically, smog allows hazy sunlight to reach the ground, but it may also be dense enough to hide aircraft flying overhead from view. Smog irritates the eyes and throat, and it can corrode structures over long periods of time.

Modern urban smog has three main toxic ingredients: nitrogen oxides, volatile organic compounds, and ozone. Nitrogen oxides and volatile organic compounds are largely automobile pollutants. Ozone forms through a photochemical reaction in the air in which nitrogen oxides react with volatile organic compounds in the presence of sunlight. Ozone is harmful to human lungs, and it aggravates bronchitis, emphysema, and asthma. Haze is a condition of the atmosphere in which aerosols obscure distant objects. Haze builds up naturally in stationary air as a result of human and natural activity. When the air is humid and abundant water vapor is available, water films grow on suspended nuclei. This creates aerosol particles large enough to obscure and scatter light, reducing visibility.
FALLOUT AND WASHOUT
Pollutants generated by a combustion process are at first carried aloft by convection. However, the larger particulates soon settle under gravity and return to the surface as fallout. Particles too small to settle out are later swept down to Earth by precipitation in a process called washout. Through a combination of fallout and washout, the atmosphere tends to be cleaned of pollutants.
Pollutants are also eliminated from the air over their source areas by wind. Strong, through-flowing winds will disperse pollutants into large volumes of cleaner air in the downwind direction. Strong winds can quickly sweep away most pollutants from an urban area, but during periods when winds are light or absent, the concentrations can rise to high values.
INVERSION AND SMOG
The concentration of pollutants over a source area rises to its highest levels when vertical mixing (convection) of the air is inhibited. This happens in an inversion—a condition in which the temperature of the air increases with altitude.
According to the adiabatic principle, a heated airparcel emerging from a smokestack or chimney will cool as it rises until it attains the same temperature as the surrounding air. In an inversion, however, the surrounding air gets warmer, not colder, with altitude. So the hot parcel will quickly arrive at the temperature of the surrounding air, and uplift will stop. Pollutants in the air parcel remain trapped below the inversion, keeping concentrations high near the ground.
Two types of inversions are important in causing high air pollutant concentrations—low-level and high-level inversions. When a low-level temperature inversion develops over an urban area with many air pollution sources, pollutants are trapped under the “inversion lid.” Heavy smog or highly toxic fog can develop. London's poisonous “pea soup” fogs of the past are an example.
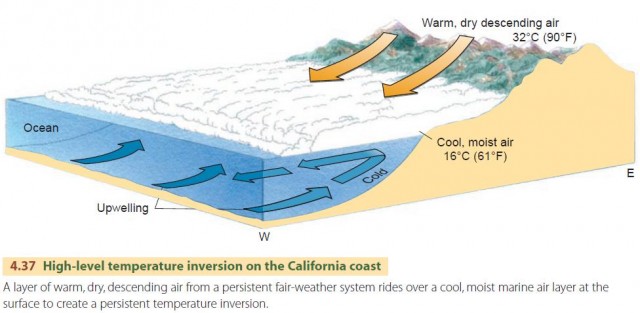
Another type of inversion is responsible for the smog problem experienced in the Los Angeles Basin and other California coastal regions, ranging north to San Francisco and south to San Diego. Here special climatic conditions produce prolonged inversions and smog accumulations (Figure 4.37). Off the California coast is a persistent fair-weather system that is especially strong in the summer. This system produces a layer of warm, dry air at upper elevations. However, a cold current of upwelling ocean bottom water runs along the coast, just offshore. Moist ocean air moves across this cool current and is chilled, creating a cool, marine air layer.
Now, the Los Angeles Basin is a low, sloping plain lying between the Pacific Ocean and a massive mountain barrier on the north and east sides. Weak winds from the south and southwest move the cool, marine air inland over the basin. Further landward movement is blocked by the mountain barrier. Since there is a warm layer above this cool marine air layer, the result is a high-level temperature inversion. Pollutants accumulate in the cool air layer and produce smog. The upper limit of the smog stands out sharply in contrast to the clear air above it, filling the basin like a lake and extending into valleys in the bordering mountains (Figure 4.38).

An actual temperature inversion, in which air temperature increases with altitude, is not essential for building a high concentration of pollutants above a city. Light or calm winds and stable air are all that are required. Stable air has a temperature profile that decreases with altitude but at a slow rate. Some convective mixing occurs in stable air, but the convective precipitation process is inhibited.
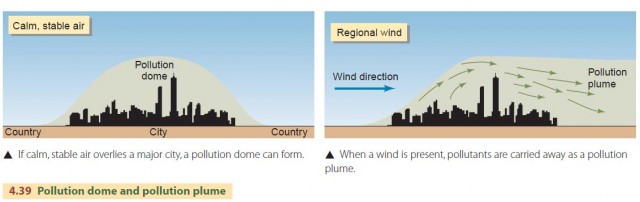
At certain times of the year, slow-moving masses of dry, stable air occupy the central and eastern portions of the North American continent. Under these conditions, a broad pollution dome can form over a city or region, and air quality will suffer (Figure 4.39). When there is a regional wind, the pollution from a large city will be carried downwind to form a pollution plume.