Electromagnetic Radiation
All surfaces—from the fiery Sun in the sky to the skin covering our bodies—constantly emit radiation. Very hot objects, such as the Sun or a light bulb filament, give off radiation that is nearly all in the form of light. Most of this energy is visible light, which we perceive with the colors of the rainbow, but the Sun also emits ultraviolet and infrared light that cannot be seen directly. Cooler objects than the Sun, such as Earth surfaces and even our own bodies, emit heat radiation. So, our planet's surface and its atmosphere constantly emit heat. Over the long run, the Earth emits exactly as much energy as it absorbs from the sun, creating a global energy balance.
Light and heat are both forms of electromagnetic radiation. You can think of electromagnetic radiation as a collection of waves, of a wide range of wavelengths, that travel away from the surface of an object. Radiant energy can exist at any wavelength. Heat and light are identical forms of electromagnetic radiation except for their wavelengths.
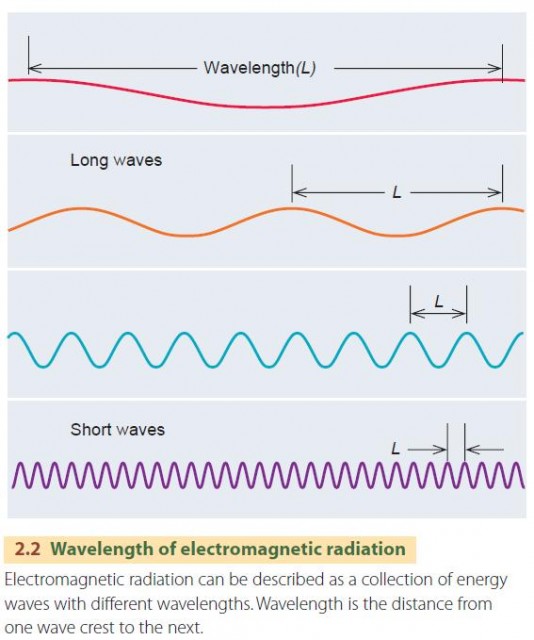
Wavelength is the distance separating one wave crest from the next wave crest, as you can see in Figure 2.2. In this book, we will measure wavelength in micrometers. A micrometer is one millionth of a meter (10–6 m). The tip of your little finger is about 15,000 micrometers wide. We use the abbreviation ?m for the micrometer. The first letter is the Greek letter ?, or mu.
Electromagnetic waves differ in wavelength throughout their entire range, or spectrum (Figure 2.3). Gamma rays and X rays lie at the short-wavelength end of the spectrum. Their wavelengths are normally expressed in nanometers. A nanometer is one one-thousandth of a micrometer, or 10–9 m, and is abbreviated nm. Gamma and X rays have high energies and can be hazardous to health. Ultraviolet radiation begins at about 10 nm and extends to 400 nm (or 0.4 ?m). It can also damage living tissues. Visible light begins at about 0.4 ?m with the color violet. Colors then gradually change through blue, green, yellow, orange, and red, until we reach the end of the visible spectrum at about 0.7 ?m. Next is nearinfrared radiation, with wavelengths from 0.7 to 1.2 ?m. This radiation is very similar to visible light—most of it comes from the Sun. We can't see near-infrared light because our eyes are not sensitive to radiation beyond about 0.7 ?m.
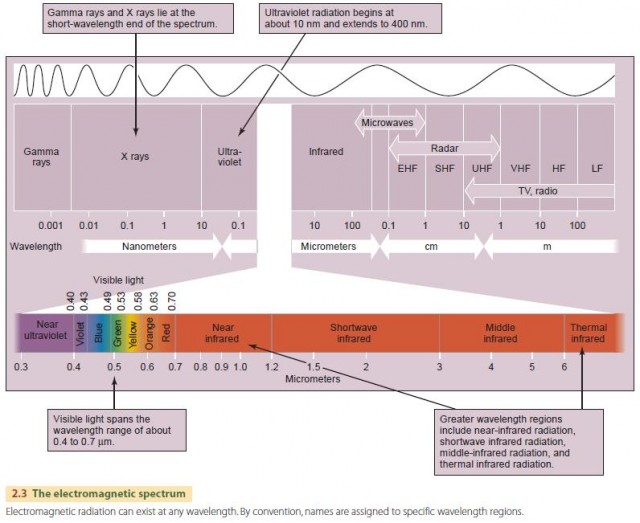
Shortwave infrared radiation also mostly comes from the Sun and lies between 1.2 and 3.0 ?m. Middle-infrared radiation, from 3.0 ?m to 6 ?m, can come from the Sun or from very hot sources on the Earth, such as forest fires and gas well flames. Next we have thermal infrared radiation, between 6 ?m and 300 ?m. This is given off by bodies at temperatures normally found at the Earth's surface. Figure 2.4 shows a thermal infrared image of a suburban scene obtained at night using a special sensor. Here red tones indicate the warmest temperatures and black tones the coldest. Windows appear red because they are warm and radiate more intensely. House walls are intermediate in temperature and appear blue. Roads and driveways are cool, as are the trees, shown in purple tones. Ground and sky are coldest (black).

RADIATION AND TEMPERATURE
There are two important physical principles to remember about the emission of electromagnetic radiation. The first is that hot objects radiate more energy than cooler objects. The flow of radiant energy from the surface of an object is directly related to the absolute temperature of the surface, measured on the Kelvin absolute temperature scale, raised to the fourth power. So if you double the absolute temperature of an object, it will emit 16 times more energy from its surface. Even a small increase in temperature can mean a large increase in the rate at which radiation is given off by an object or surface. The second principle is that the hotter the object, the shorter are the wavelengths of radiation that it emits. This inverse relationship between wavelength and temperature means that very hot objects like the Sun emit radiation at short wavelengths. Because the Earth is a much cooler object, it emits radiation with longer wavelengths. This principle explains why the Sun emits light and the Earth emits heat.
SOLAR RADIATION
Our Sun is a ball of constantly churning gases that are heated by continuous nuclear reactions. It is about average in size compared to other stars, and it has a surface temperature of about 6000°C (about 11,000°F). The Sun's energy travels outward in straight lines or rays at a speed of about 300,000 km (about 186,000 mi) per second— the speed of light. At that rate, it takes the energy about 8 ? minutes to travel the 150 million km (93 million mi) from the Sun to the Earth.
The rays of solar radiation spread apart as they move away from the Sun. This means that a square meter on Mars will intercept less radiation than on Venus because Mars lies farther from the Sun. The Earth only receives about one-half of one-billionth of the Sun's total energy output.
Solar energy is generated by nuclear fusion reactions inside the Sun, as hydrogen is converted to helium at very high temperatures and pressures. A vast quantity of energy is generated this way, which finds its way to the Sun's surface. The rate of solar energy production is nearly constant, so the output of solar radiation also remains nearly constant, as does the amount of solar energy received by the Earth. The rate of incoming energy, known as the solar constant, is measured beyond the outer limits of the Earth's atmosphere, before any energy has been lost in the atmosphere.
You've probably seen the watt (W) used to describe the power, or rate of energy flow, of a light bulb or other home appliance. When we talk about the intensity of received (or emitted) radiation, we must take into account both the power of the radiation and the surface area being hit by (or giving off) energy. So we use units of watts per square meter (W/m2). The solar constant has a value of about 1367 W/m2. Because there are no common equivalents for this energy flow rate in the English system, we will use only metric units.
CHARACTERISTICS OF SOLAR ENERGY
Let's look in more detail at the Sun's output as it is received by the Earth (illustrated in Figure 2.5). Energy intensity is shown on the graph on the vertical scale. Note that it is a logarithmic scale—that is, each whole unit marks an intensity 10 times greater than the one below. Wavelength is shown on the horizontal axis, also on a logarithmic scale.
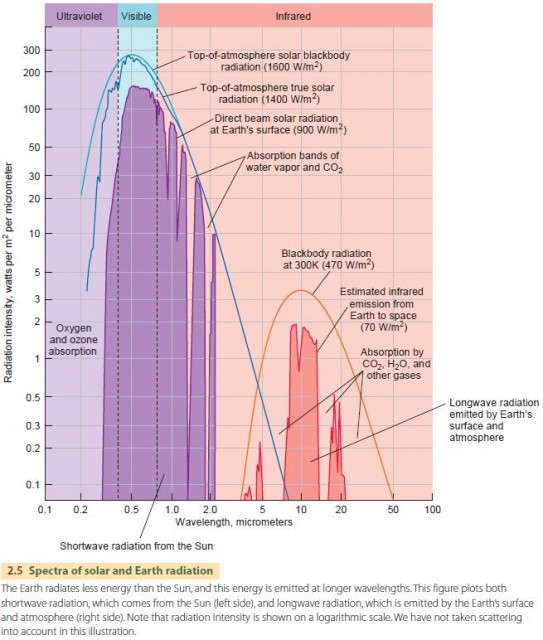
The left side of Figure 2.5 shows how the Sun's incoming electromagnetic radiation varies with wavelength. The uppermost line indicates how a “perfect” Sun would supply solar energy at the top of the atmosphere. By “perfect,” we mean a Sun at a temperature of 6000 K radiating as a blackbody—an ideal surface that follows physical theory exactly. The solid line shows the actual output of the Sun as measured at the top of the atmosphere. It is quite close to the “perfect” Sun, except for ultraviolet wavelengths, where the real Sun emits less energy. The Sun's output peaks in the visible part of the spectrum. We can see that human vision is adjusted to the wavelengths where solar light energy is highest.
The solar radiation actually reaching the Earth's surface is quite different from the solar radiation measured above the Earth's atmosphere. This is because solar radiation is both absorbed and scattered by varying amounts at different wavelengths as it passes through the atmosphere. Molecules and particles in the atmosphere intercept and absorb radiation at particular wavelengths. This atmospheric absorption directly warms the atmosphere in a way that affects the global energy balance, as we will discuss toward the end of this chapter. Solar rays can also be scattered into different directions when they collide with molecules or particles in the atmosphere. Rays can be diverted back up into space or down toward the surface, and may be scattered several times.
Solar energy received at the surface ranges from about 0.3 ?m to 3 ?m. This is known as shortwave radiation. We will now turn to the longer wavelengths of energy that are emitted by the Earth and atmosphere.
LONGWAVE RADIATION FROM THE EARTH
Remember that both the range of wavelengths and the intensity of radiation emitted by an object depend on the object's temperature. Because the Earth's surface and atmosphere are much colder than the Sun, our planet radiates less energy than the Sun and this energy is emitted at longer wavelengths.
The right side of Figure 2.5 shows exactly that. The upper line shows the radiation of a blackbody at a temperature of about 300 K (23°C, 73°F), which is a good approximation for the Earth as a whole. At this temperature, radiation ranges from about 3 to 30 ?m and peaks at about 10 ?m in the thermal infrared region. This thermal infrared radiation emitted by the Earth is longwave radiation.
Beneath the blackbody curve is an irregular series of peaks that show upwelling energy emitted by the Earth and atmosphere as measured at the top of the atmosphere. Some wavelengths in this range seem to be missing, especially between 6–8 ?m, 14–17 ?m, and above 21 ?m. These wavelengths are almost completely absorbed by the atmosphere before they can escape. Water vapor and carbon dioxide are the main absorbers, playing a large part in the greenhouse effect, which we will discuss shortly.
There are still three regions where outgoing energy flow from the Earth to space is significant—4 to 6 ?m, 8 to 14 ?m, and 17 to 21 ?m. We call these windows through which longwave radiation leaves the Earth and flows to space.
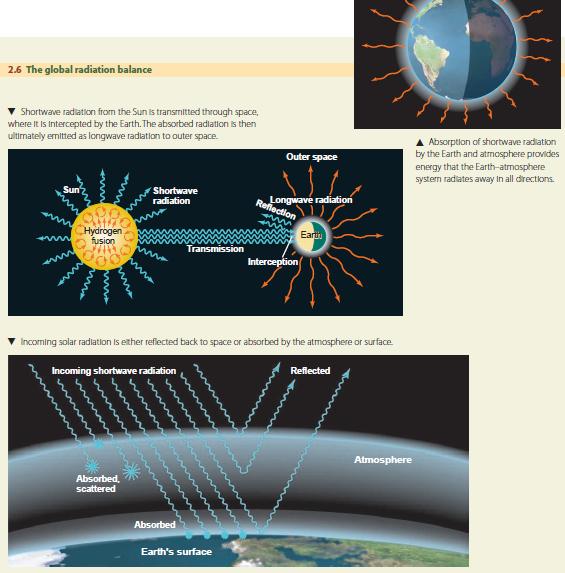
THE GLOBAL RADIATION BALANCE
The Earth constantly absorbs solar shortwave radiation and emits longwave radiation. Figure 2.6 presents a diagram of this energy flow process, which we refer to as the Earth's global radiation balance. The Sun provides a nearly constant flow of shortwave radiation that is intercepted by the Earth. Scattering reflects part of this radiation back into space without absorption. The remaining energy is absorbed by atmosphere, land, or ocean, and ultimately emitted as longwave radiation to space. In the long run, absorbed incoming radiation is balanced by emitted outgoing radiation. Since the temperature of a surface is determined by the amount of energy it absorbs and emits, the Earth's overall temperature tends to remain constant.