What Controls Wavelengths of Radiation?
ELECTROMAGNETIC RADIATION takes many different forms, each of which have very different properties, determined by their wavelengths. Variations in wavelength explain the existence of different colors and warming of the Earth due to climate change. How are differences in the wavelength of electromagnetic energy (EMR) expressed in our world and in the Solar System?
What Range of Wavelengths Does an Object Emit?
Not all molecules in an object vibrate or move at the same speed, so an object emits energy with some variation in the wavelengths and amounts of energy it emits. Some molecules vibrate or move faster, emitting EMR with shorter wavelengths and higher energies. Other molecules vibrate or move more slowly, resulting in EMR with longer wavelengths and lower energies. Temperature is a measure of the average energy content of all molecules in an object.
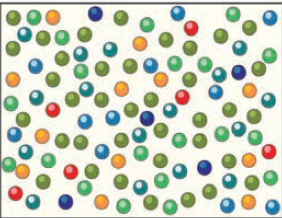
Molecules in a cubeshaped container are color coded by energy level; red is lowest, purple is highest, and other colors are in between. Molecules possessing the average energy level (i.e., temperature of the object) are in olive green. Observe the relative proportions of the different energy levels (that is, the different amounts of each color of molecule). Which are most abundant and which are least abundant?
This graph shows the number of molecules for each class of energy in the square object we just examined. The height of each column represents the number of molecules emitting energy at that wavelength.
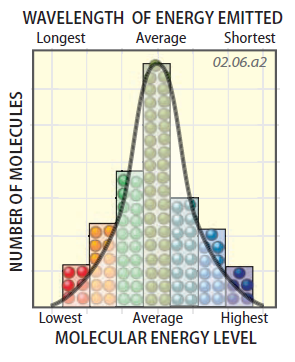
The peak of the graph represents the wavelengths and energy levels that are most common. Columns to the left of the peak have lower energy and longer wavelengths than the peak, whereas those to the right have higher energy and shorter wavelengths. Taking into account all the molecules, there will be an average energy level emitted by this material at this temperature (near the peak).
What Type of Energy Is Emitted from an Object?
The rate of motion of molecules within an object is related to the object's overall temperature. There is also a relationship between temperature and the wavelength of energy emitted by that object.
1. Examine the figure below, which shows three blocks of the same material but at different temperatures, with the blue block being cool, the brown block being warmer, and the red block being the hottest. Coming off each block are arrows depicting the amount and wavelength of EMR being emitted. Observe how the amounts and wavelengths relate to temperature.
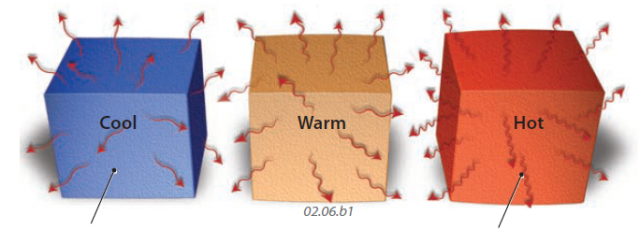
2. If an object is relatively cool, the motion of its constituent atoms and molecules will be relatively slow. This in turn causes the rate of energy shifts to be less frequent, resulting in energy with a longer wavelength.
3. As the temperature of an object increases, it not only emits more EMR (as shown by more arrows for the warm and hot blocks in the figure), but the wavelength of the EMR becomes shorter at higher temperatures. In a hot object, the atoms and molecules are moving relatively fast, and they change energy states more often, producing EMR with a shorter wavelength. If you shake a rope rapidly, you make shorter waves than if you shake it slowly. Go try it!
4. Whether an object is cool or hot, it emits a range of wavelengths of EMR, as described in the section above. However, scientists discovered a numeric relationship relating the dominant wavelength of EMR to temperature, a relationship known as Wien's Law.
5. The red curve on this graph represents Wien's Law, plotting the dominant wavelength of EMR emitted as a function of temperature. The curve is highest on the left, indicating that an object with a low temperature emits EMR with very long wavelengths.
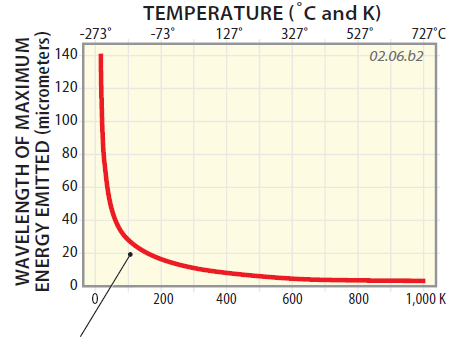
6. As the temperature of an object increases, the wavelength decreases very rapidly at first and then more slowly toward higher temperatures. Wien's Law indicates that cold objects emit much longer wavelength energy that hot objects. Using this graph or the equation associated with Wien's Law, we can use the temperature of an object to then predict the dominant wavelength of EMR it emits. For the Sun's very high temperatures (off the curve to the right), we predict energy wavelengths of 0.5 micrometers. The Earth's much cooler temperature yields longer wavelengths of about 10 micrometers.
What Is the Electromagnetic Radiation Spectrum?
The various objects in the universe emit a diversity of wavelengths of electromagnetic radiation. EMR varies from relatively long-wavelength, low-energy radio waves, which bombard our bodies every second of every day, to shortwavelength, high-energy X-rays and gamma rays, which are not so benign. The different wavelengths (and therefore frequencies) of electromagnetic radiation are arranged in what is called the electromagnetic spectrum, shown below. Inspect this figure and then read the associated text. In this figure, the longer wavelengths are at the top.
1. The longest wavelengths of EMR are radio waves. These include the waves that carry signals from typical FM and AM radio stations, and also include even longer radio waves called VLF (for very low frequency), with wavelengths of up to 100 km. VLF waves can penetrate significant depths of water and so are used for communication with submarines.
2. Microwaves are another form of EMR, with shorter wavelengths, higher frequencies, and higher energies than radio waves. Microwave ovens use a specific frequency of microwave that energetically excites (heats) water molecules.
3. Next on the scale is infrared energy (IR). Although we cannot see IR, our skin is sensitive to it, so we often think of infrared radiation as heat. Infrared energy is incredibly important on Earth, playing a key role in keeping our planet a hospitable temperature. There are several types of IR, including thermal-IR, which is close to microwaves in wavelength, and nеаr-IR, which is near to visible light, the next entry on the spectrum.
4. Visible light occupies a relatively narrow part of the spectrum. It varies from red colors at long wave-lengths to violet colors at short ones. Orange, yellow, green, and blue are in between red and violet.
5. Next to, and with shorter wavelengths (higher frequencies) and more energy than visible light, is ultraviolet light (UV). Ultraviolet is more energetic than visible light and is known to cause skin cancers and possible genetic mutations.
6. The shortest wavelength (highest frequency and therefore most energetic) waves are X-rays and gamma rays. These are potentially harmful. The energy in X-rays is used in medical technology as it will pass through soft tissue, but not bone.

7. The different colors of visible light are different wavelengths of energy, ranging between 0.4 pm (violet) and 0.7 pm (red). The component wavelengths of visible light can be seen when the light is split by an optical prism or in a rainbow (A).
8. The human eye is sensitive to radiation of the wavelengths between violet and red, and this is why this portion of the EMR spectrum is known as “visible light.”The Sun's wavelengths are concentrated at 0.5 pm, where we predicted them based on Wien's Law. This wavelength is in the middle of visible light, coinciding with blue light. It is not a coincidence that the human eye developed to detect EMR of the wavelengths that carry most energy from the Sun.
9. The Sun produces huge quantities of energy per square meter of its surface, as indicated by the yellow area on the graph to the right. It also produces most of its energy near 0.5 micrometers, centered on the wavelength of visible light; these wavelengths are called shortwave radiation. The Sun emits a very low proportion of its energy below 0.1 micrometers and above 1.0 micrometers in wavelength.
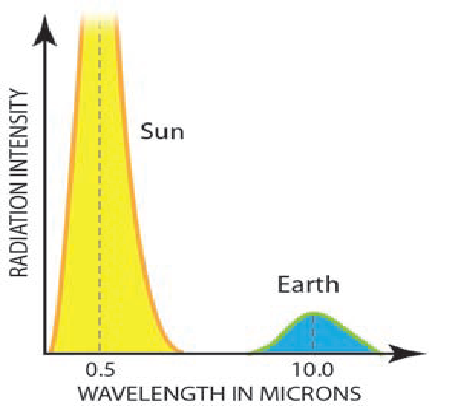
10. The Earth emits low quantities of energy, as indicated by the relatively small blue area on the graph, which is here greatly exaggerated relative to the curve for the Sun. Most of that energy is emitted back to space at wavelengths of around 10 micrometers, called longwave radiation. As we will discover, the fact that Earth receives its energy at short wavelengths, and returns it at wavelengths 20 times longer, is of fundamental importance to maintaining temperatures on Earth favorable for life.