Composition of the Atmosphere
The Earth is surrounded by air—a mixture of various gases that reaches up to a height of many kilometers. This envelope of air makes up our atmosphere (Figure 2.13). It is held in place by the Earth's gravity. Almost all the atmosphere (97 percent) lies within 30 km (19 mi) of the Earth's surface. The upper limit of the atmosphere is at a height of approximately 10,000 km (about 6000 mi) above the Earth's surface—a distance that is nearly as large as Earth's diameter.
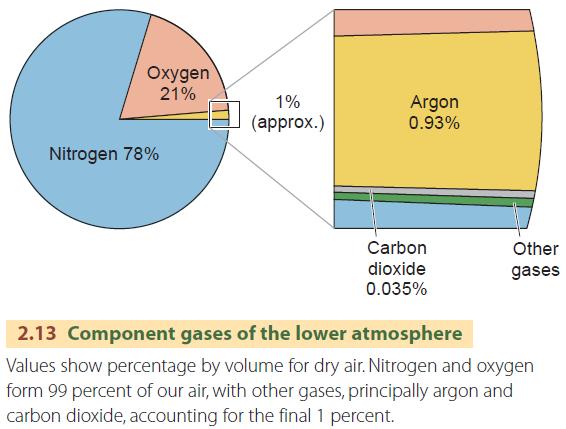
The proportion of gases in dry air is highly uniform up to an altitude of about 80 km (50 mi). About 99 percent of pure, dry air is nitrogen (about 78 percent by volume) and oxygen (about 21 percent). These two main component gases of the lower atmosphere are perfectly mixed, so pure, dry air behaves as if it is a single gas with very definite physical properties.
Nitrogen gas is a molecule consisting of two nitrogen atoms (N2). It does not easily react with other substances. Soil bacteria do take up very small amounts of nitrogen, which can be used by plants, but otherwise nitrogen is largely a “filler,” adding inert bulk to the atmosphere. In contrast, oxygen gas (O2) is chemically very active, combining readily with other elements in the process of oxidation. Fuel combustion is a rapid form of oxidation, while certain types of rock decay (weathering) are very slow forms of oxidation. Living tissues require oxygen to convert foods into energy.
The remaining 1 percent of dry air is mostly argon, an inactive gas of little importance in natural processes, with a very small amount of carbon dioxide (CO2), amounting to about 0.0385 percent. Although the amount of CO2 is small, it is a very important atmospheric gas because it absorbs much of the incoming shortwave radiation from the Sun and outgoing longwave radiation from the Earth. The greenhouse effect is caused when longwave radiation is absorbed by CO2 molecules in the lower atmosphere, which reradiate some of that heat back to the surface.
Carbon dioxide is also used by green plants, which convert it to its chemical compounds to build up their tissues, organs, and supporting structures during photosynthesis. Water vapor is another important atmospheric gas. Individual water vapor molecules mix freely with other atmospheric gases, but water vapor can vary highly in concentration. Water vapor usually makes up less than 1 percent of the atmosphere, but under very warm, moist conditions, as much as 2 percent of the air can be water vapor. Since it is a good absorber of heat radiation, like carbon dioxide, it plays a major role in warming the lower atmosphere and enhancing the greenhouse effect.
Another small, but important, constituent of the atmosphere is ozone, which we described in our Eye on Global Change opening feature. Ozone in the upper atmosphere is beneficial because it shields life at the Earth's surface from harmful solar ultraviolet radiation. But in the lowest layers of the atmosphere, ozone is an air pollutant that damages lung tissue and aggravates bronchitis, emphysema, and asthma.