Energy and Matter Flow in Ecosystems
This chapter is the first of two chapters that look at biogeography. Biogeography focuses on the distribution of plants and animals—the biota—over the Earth. It identifies and describes the processes that influence plant and animal distribution patterns. Ecological biogeography looks at how the distribution patterns of organisms are affected by the environment. Historical biogeography focuses on how spatial distribution patterns of organisms arise over time and space.
Before we turn to those processes, we must begin by looking at some ideas from the domain of ecology, which is the science of the interactions among life-forms and their environment. These ideas concern how organisms live and interact as ecosystems and how energy and matter are cycled by ecosystems.
The term ecosystem refers to a group of organisms and their environment. Ecosystems take up matter and energy as plants and animals grow, reproduce, and maintain life. That matter and energy can be recycled within the ecosystem or exported out of the ecosystem. Ecosystems balance the various processes and activities within them. Many of these balances are robust and self-regulating, but some are quite sensitive and can be easily upset or destroyed.
THE FOOD WEB
Energy is transferred through an ecosystem in steps, making up a food chain or a food web. At the bottom of the chain are the primary producers, which absorb sunlight and use the light energy to convert carbon dioxide and water into carbohydrates (long chains of sugar molecules) and eventually into other biochemical molecules, by photosynthesis.
The primary producers support the consumers—organisms that ingest other organisms as their food source. Finally, decomposers feed on decaying organic matter, from all levels of the web. Decomposers are largely microscopic organisms (microorganisms) and bacteria.
The food web is really an energy flow system, tracing the path of solar energy through the ecosystem. Solar energy is absorbed by the primary producers and stored in the chemical products of photosynthesis. As these organisms are eaten and digested by consumers, chemical energy is released. This chemical energy is used to power new biochemical reactions, which again produce stored chemical energy in the consumers' bodies.
Energy is lost at each level in the food web through respiration. You can think of this lost energy as fuel burned to keep the organism operating. Energy expended in respiration is ultimately lost as waste heat and cannot be stored for use by other organisms higher up in the food chain. This means that, generally, both the numbers of organisms and their total amount of living tissue must decrease greatly up the food chain. In general, only 10 to 50 percent of the energy stored in organic matter at one level can be passed up the chain to the next level. Normally, there are about four levels of consumers.
The number of individuals of any species present in an ecosystem depends on the resources available to support them. If these resources provide a steady supply of energy, the population size will normally stay steady. But resources can vary with time, for example, in an annual cycle. In those cases, the population size of a species depending on these resources may fluctuate in a corresponding cycle.
PHOTOSYNTHESIS AND RESPIRATION
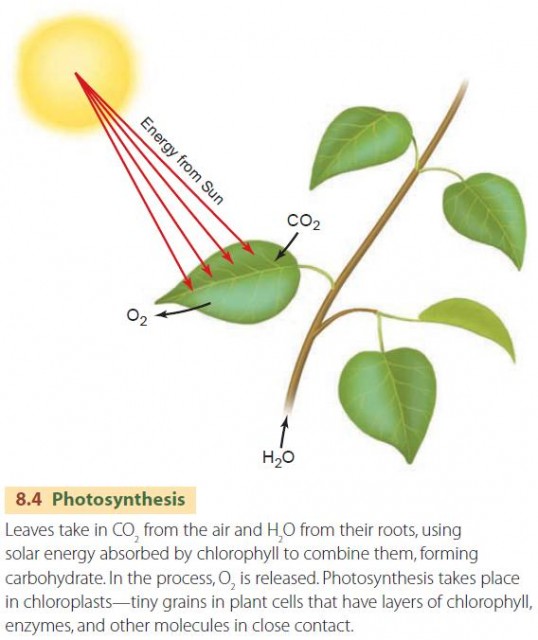
Simply put, photosynthesis is the production of carbohydrate (Figure 8.4). Carbohydrate is a general term for a class of organic compounds that are made from the elements carbon, hydrogen, and oxygen. Carbohydrate molecules are composed of short chains of carbon bonded to one another. Hydrogen (H) atoms and hydroxyl (OH) molecules are also attached to the carbon atoms. We can symbolize a single carbon atom with its attached hydrogen atom and hydroxyl molecule as –CHOH–. The leading and trailing dashes indicate that the unit is just one portion of a longer chain of connected carbon atoms. Photosynthesis of carbohydrate requires a series of complex biochemical reactions using water (H2O) and carbon dioxide (CO2 ) as well as light energy. This process requires chlorophyll, a complex organic molecule that absorbs light energy for use by the plant cell. A simplified chemical reaction for photosynthesis can be written as:
H2O + CO2 + light energy ? –CHOH– + O2
Oxygen gas molecules (O2) are a by-product of photosynthesis. Because gaseous carbon as CO2 is “fixed” to a solid form in carbohydrate, we also call photosynthesis a carbon fixation process.
Respiration is the opposite of photosynthesis. In this process, carbohydrate is broken down and combines with oxygen to yield carbon dioxide and water. The overall reaction is:
–CHOH– + O2 ? CO2 + H2O + chemical energy
As with photosynthesis, the actual reactions involved are not this simple. The chemical energy released is stored in several types of energy-carrying molecules in living cells and used later to synthesize all the biological molecules used to sustain life.
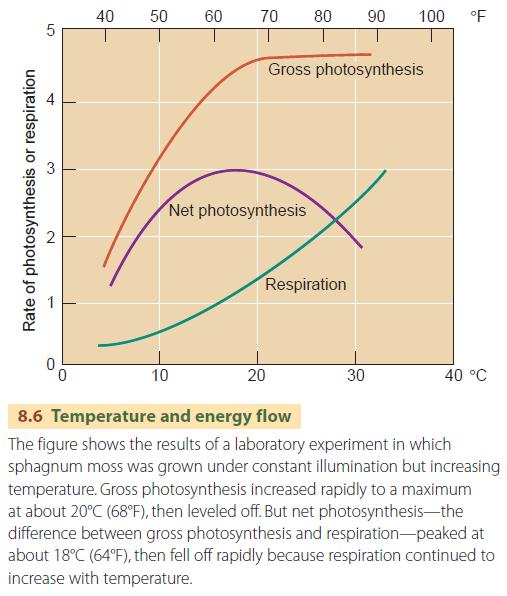
We have to take respiration into account when talking about the amount of new carbohydrate placed in storage. Gross photosynthesis is the total amount of carbohydrate produced by photosynthesis. Net photosynthesis is the amount of synthesized carbohydrate remaining after respiration has broken down sufficient carbohydrate to power the plant:
Net photosynthesis = Gross photosynthesis ? Respiration
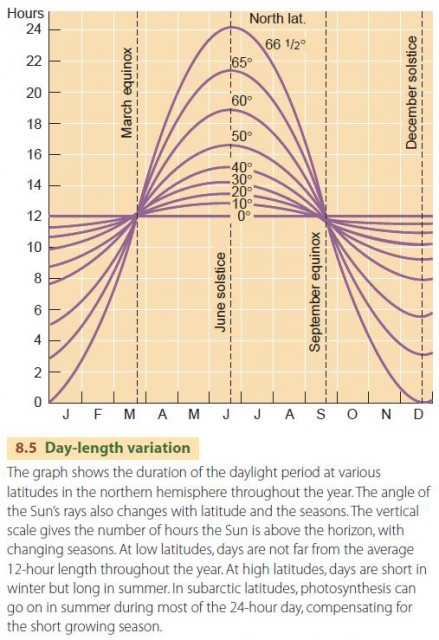
The rate of net photosynthesis depends on the intensity of light energy available, up to a limit. Most green plants only need about 10 to 30 percent of full summer sunlight for maximum net photosynthesis. Once the intensity of light is high enough for maximum net photosynthesis, the duration of daylight becomes an important factor in determining the rate at which the products of photosynthesis build up in plant tissues (Figure 8.5). The rate of photosynthesis also increases as air temperature increases, up to a limit (Figure 8.6).
NET PRIMARY PRODUCTION
Plant ecologists measure the accumulated net production by photosynthesis in terms of biomass, which is the dry weight of organic matter. This quantity could, of course, be stated for a single plant or animal, but a more useful measurement is the biomass per unit of surface area within the ecosystem—that is, grams of biomass per square meter or (metric) tons of biomass per hectare (1 hectare = 104 m2). Of all ecosystems, forests have the greatest biomass because of the large amount of wood that the trees accumulate through time. The biomass of grasslands and croplands is much smaller in comparison. The biomass of freshwater bodies and the oceans is about one-hundredth that of the grasslands and croplands. The amount of biomass per unit area tells us about the amount of photosynthetic activity, but it can be misleading.
In some ecosystems, biomass is broken down very quickly by consumers and decomposers. So if we want to know how productive the ecosystem is, it's better to work out the annual yield of useful energy produced by the ecosystem, or the net primary production. Net primary production represents a source of renewable energy derived from the Sun that can be exploited to fill human energy needs. The use of biomass as an energy source involves releasing solar energy that has been fixed in plant tissues through photosynthesis. It can take place in a number of ways—by burning wood for fires, for example.
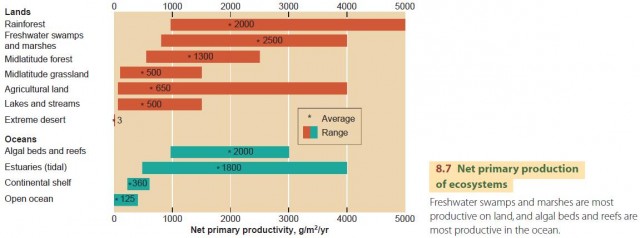
Figure 8.7 shows the net primary production of various ecosystems in units of grams of dry organic matter produced annually from one square meter of surface. The highest values are in two quite unlike environments: forests and wetlands (swamps, marshes, and estuaries). Agricultural land compares favorably with grassland, but the range is very large in agricultural land, reflecting many factors such as availability of soil water, soil fertility, and use of fertilizers and machinery.
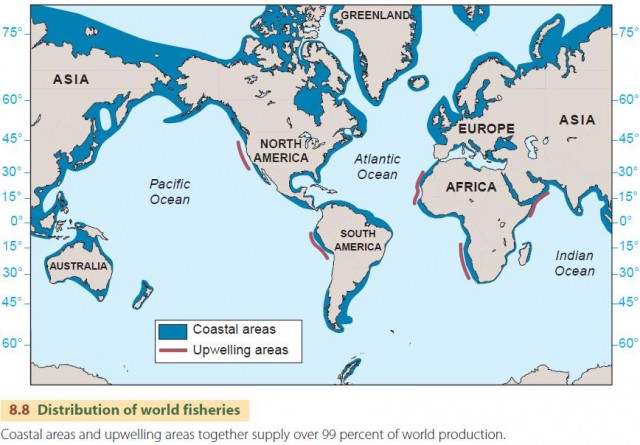
Open oceans aren't generally very productive. Continental shelf areas are better, supporting much of the world's fishing industry (Figure 8.8). Upwelling zones are also highly productive.
THE CARBON CYCLE
We've seen how energy from the Sun flows through ecosystems, passing from one part of the food chain to the next. Ultimately, that energy is radiated to space and lost from the biosphere. Matter also moves through ecosystems, but because gravity keeps surface material earthbound, matter can't be lost in the global ecosystem. As molecules are formed and re-formed by chemical and biochemical reactions within an ecosystem, the atoms that compose them are not changed or lost. In this way, matter is conserved, and atoms and molecules are used and reused, or cycled, within ecosystems.
Atoms and molecules move through ecosystems under the influence of both physical and biological processes. We call the pathways that a particular type of matter takes through the Earth's ecosystem a biogeochemical cycle (sometimes referred to as a material cycle or nutrient cycle).
The major features of a biogeochemical cycle are diagrammed in Figure 8.9. Any area or location of concentration of a material is a pool. There are two types of pools: active pools, where materials are in forms and places easily accessible to life processes, and storage pools, where materials are more or less inaccessible to life.
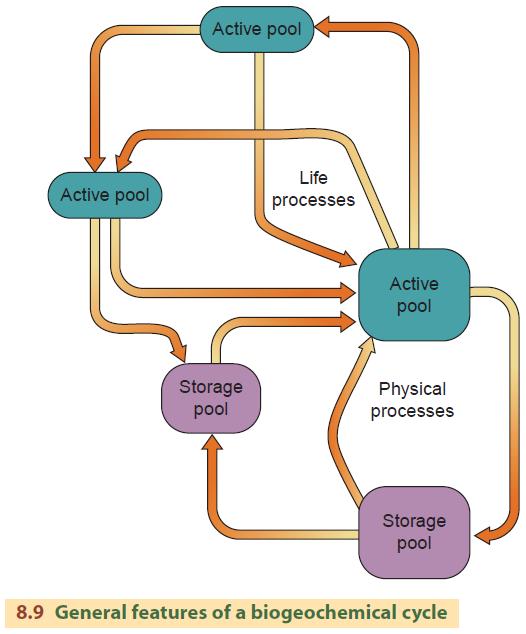
A system of pathways of material flows connects the various active and storage pools within the cycle. Pathways can involve the movement of material in all three states of matter—gas, liquid, and solid. For example, carbon moves freely in the atmosphere as carbon dioxide gas and freely in water as dissolved CO2 and as carbonate ion (CO3 = ). It also takes the form of a solid in deposits of limestone and dolomite (calcium and magnesium carbonate).
Ecologists have studied and documented biogeochemical cycles for many elements, including carbon, oxygen, nitrogen, sulfur, and phosphorus. Of these, the carbon cycle is probably the most important. That's because all life is composed of carbon compounds of one form or another and human activities are modifying the carbon cycle in significant ways.
Figure 8.10 looks at this process in more detail. Atmospheric carbon dioxide makes up less than 2 percent of all the carbon. The atmospheric pool is supplied by plant and animal respiration in the oceans and on the lands, by outgassing volcanoes, and by fossil fuel combustion in industry and power generation. The atmosphere loses carbon to oceanic and terrestrial photosynthesis.
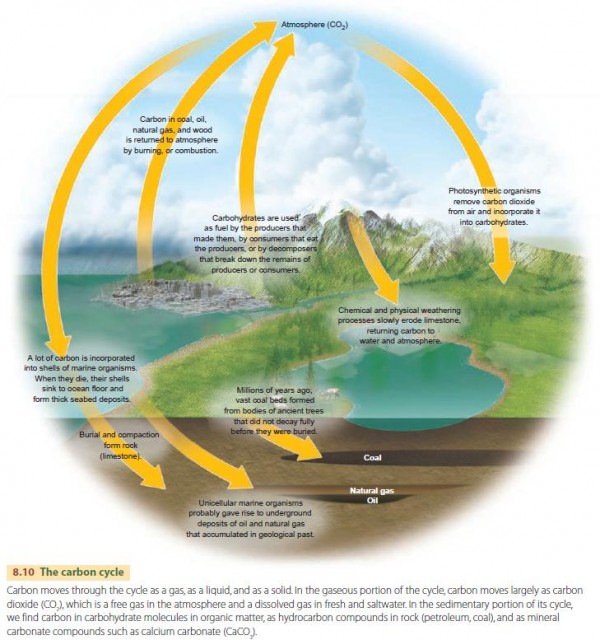
As noted earlier in this chapter, humans are affecting the carbon cycle by burning fossil fuels. CO2 is being released to the atmosphere at a rate far beyond that of any natural process, causing global warming. Another important human impact lies in changing the Earth's land covers—for example, in clearing forests or abandoning agricultural areas, or in letting agricultural areas grow back to forests or rangelands.
THE NITROGEN CYCLE
The nitrogen cycle is another important biogeochemical cycle. Nitrogen makes up 78 percent of the atmosphere by volume, so the atmosphere is a vast storage pool in this cycle. Figure 8.11 diagrams the nitrogen cycle. Nitrogen as N2 in the atmosphere can't be assimilated directly by plants or animals. But certain microorganisms, including some soil bacteria and blue-green algae, can change N2 into useful forms in a process called nitrogen fixation. Legumes—such as clover, alfalfa, soybeans, peas, beans, and peanuts—are also able to fix nitrogen, with help from bacteria. They have a symbiotic relationship with bacteria of the genus Rhizobium, which is associated with some 190 species of trees and shrubs. The bacteria infect these plants' root cells and supply nitrogen to the plant through nitrogen fixation, while the plants supply nutrients and organic compounds needed by the bacteria. Crops of legumes are often planted in seasonal rotation with other food crops to ensure an adequate nitrogen supply in the soil. Other soil bacteria convert nitrogen from usable forms back to N2, in a process called denitrification that returns the nitrogen to the atmosphere. Other processes shown in the figure are ammonification, nitrification, and assimilation.
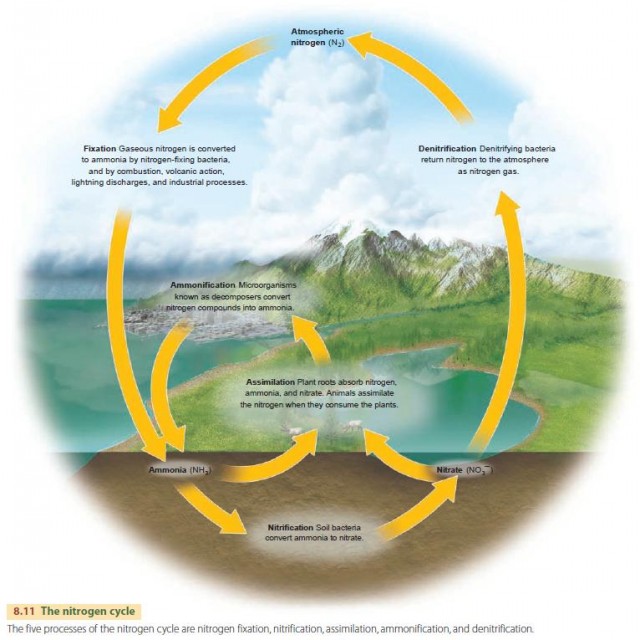
At the present time, nitrogen fixation far exceeds denitrification, thanks to human activity. We fix nitrogen in the manufacture of nitrogen fertilizers; by oxidizing nitrogen in the combustion of fossil fuels; and through the widespread cultivation of legumes. At present rates, nitrogen fixation from human activity nearly equals all natural biological fixation, and usable nitrogen is accumulating in the Earth's ecosystems.
Much of this newly fixed nitrogen is carried from the soil into rivers and lakes and ultimately to the ocean, causing water pollution. The nitrogen stimulates the growth of algae and phytoplankton, which in turn reduce quantities of dissolved oxygen through respiration. Oxygen then drops to levels that are too low for many desirable forms of aquatic life. These problems will be accentuated in years to come because industrial fixation of nitrogen in fertilizer manufacture is doubling about every six years at present. The global impact of such large amounts of nitrogen reaching rivers, lakes, and oceans on the Earth's global ecosystem remains uncertain.