How Does Water Occur in the Atmosphere?
THE PRESENCE AND ABUNDANCE OF WATER in the atmosphere are a fundamental control of weather and climate, which both have a profound influence on our lives. The molecular structure of water causes it to have special properties that we can observe every day and that are important to life on Earth. In what forms does water occur in the atmosphere, and how did the water get there?
How Is Water Expressed In and Near the Atmosphere?
Examine this photograph of the Himalaya in Tibet and identify all the places where there are visible expressions of water. Are there places where water is likely to be present but not visible? Ponder this for a moment before reading on.

Water in the atmosphere, as on the Earth's surface and in its subsurface, occurs in three forms: as a gas (water vapor), a liquid (liquid water), and a solid (ice). In this scene, liquid water in the atmosphere is expressed as tiny drops in the clouds and as any raindrops falling from the clouds. Ice crystals, expressed as snow, are on the surface, but are also present as small crystals in some of the clouds. Water vapor is also present, but is not obvious — it always occurs as an invisible gas. Clouds, like the rest of the atmosphere, contain water vapor but only the airborne drops and ice crystals are visible.
Tiny water drops also form fog and mist, such as on the Galapagos Islands. Such moisture sustains unusual plants that extract moisture directly from the atmosphere or from the liquid water (dew) that forms on leaves and other hard surfaces, including on the back of the Giant Tortoise (~1 m across) grazing on vegetation sustained by the mist.

What Are Some Important Properties of Water?
Recall that water (H2O) is a molecule composed of one oxygen atom strongly bonded to two hydrogen atoms. The asymmetrical arrangement of the hydrogen atoms causes the molecule to have a positive charge on the side with the hydrogen atoms and a negative charge on the opposite side, near the oxygen atom. A molecule with this charge distribution is said to be polar, and the polar nature of water is why it is such a good solvent (can dissolve other substances). This polar character has many other implications.
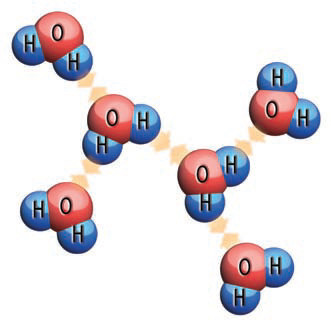
The positive side of one water molecule is attracted to the negative side of an adjacent water molecule, forming a weak bond (i.e., a hydrogen bond) that tends to keep adjacent water molecules together. This attraction is what causes water to tend to stay together as a discrete drop, rather than flowing away. This tendency for water to stay together with a discrete outer surface is called surface tension. Surface tension must be overcome to allow small drops of water to form in the atmosphere, as in clouds, and to allow these small drops to combine into larger drops, forming a raindrop. Surface tension also has to be overcome during evaporation, because it tends to hold water molecules within the liquid rather than letting them escape into the air.

Surface tension allows water to attach itself to other objects, such as this damp cloth. Note that the moisture has climbed up the cloth, higher than the level of the water. This ability of water to travel upward within small spaces is called capillary action. Capillary action is important in the upward motion of liquid water in soil, drawing soil water up toward the surface where it can evaporate. It is also important in plants, allowing water to rise from the roots through the branches and into the leaves.

How Do Water Molecules Move Between Liquid Water and Water Vapor?
Water molecules move between the three states — gas, liquid, and solid — in response to changes in the energy of the system, mostly changes in thermal energy from insolation (or lack thereof). What actually occurs during such changes in state at the level of individual molecules? Here we take a closer look at the movement of water molecules between liquid and vapor, focusing on how the energy levels of the molecules instigate change.
1. This figure shows a totally closed container of water and air, with water molecules in each state color coded for their energy levels, with yellow, orange, and red representing low energy levels, and green, blue, and purple representing higher energy levels. Based on the colors in the container, molecules of water vapor (in the air) tend, on average, to have higher energy levels than water molecules in the liquid.
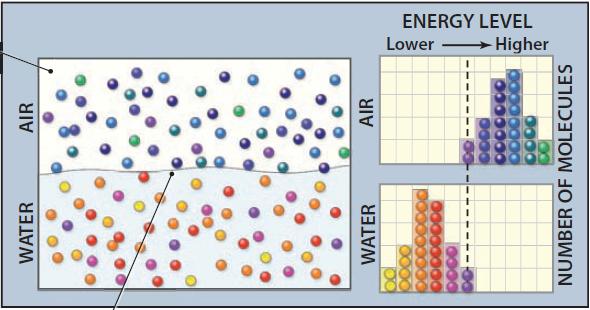
2. Note, however, that some molecules in the air have similar energy levels (purplish red on this figure) to those in the water. If the energy levels of vapor molecules are sufficiently low, these low-energy molecules will condense and join the liquid. Similarly, some molecules of the liquid will have sufficient energy to evaporate and pass into the vapor phase.
3. The graphs (histograms) on the right side of the figure display the frequency of molecules at various energy levels in the air (upper histogram) and water (lower histogram). In general, energy levels are higher in the air, as expressed by the peak of the air histogram being farther to the right (toward higher energy levels) than the peak for the water (liquid) histogram. This higher energy of the vapor is largely because of the latent heat of vaporization, which is energy the molecules gained primarily from insolation as they moved from liquid to vapor states. The critical energy level where changes in state (evaporation or condensation) occur is indicated by the dashed, vertical gray line common to both graphs. This system is in equilibrium when as many molecules are changing from liquid to gas as are moving in the opposite direction.
4. Due to the overlap in energies of molecules in the liquid and vapor, molecules are constantly moving from one state to the other. This figure illustrates that some molecules in the liquid attain high enough energy states to escape the water surface and become a molecule of water vapor (evaporation). In contrast, some vapor molecules will drop low enough in energy levels that they will join the liquid (condensation). In equilibrium there is an equal exchange of water molecules between the liquid and vapor.
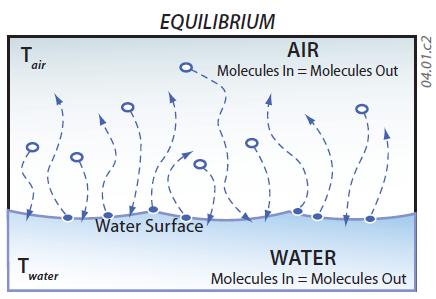
5. If the entire system is at higher overall energy levels, such as when the water and air are heated by the Sun or on a stove, many molecules in the liquid become more energetic, reaching energy levels high enough to allow them to escape into the air (i.e., evaporate). Fewer gas molecules in the air have low enough energy levels to condense into liquid. As a result of an increase in the energy of the system, increased evaporation causes the number of gas molecules (water vapor) to increase, while the liquid water loses mass.
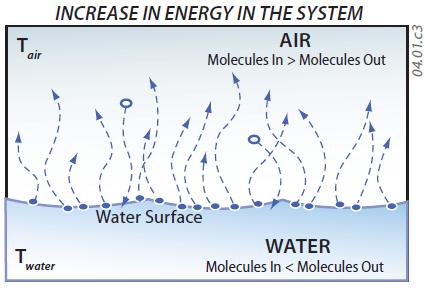
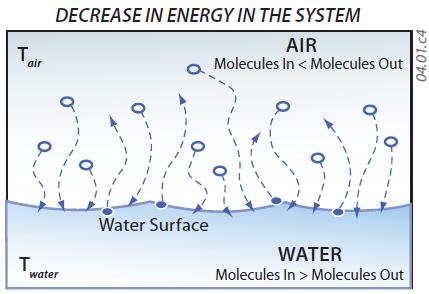
6. If the system has lower overall energy levels, as when it is cooled, more gas molecules condense into liquid, while fewer molecules in the liquid evaporate. Since more water molecules condense onto the water surface than evaporate from it, the liquid gains mass. In contrast, the gas loses water molecules, causing a decrease in the amount of water vapor in the air. This transfer from vapor to liquid occurs when water drops condense on the outside of a cold glass or beverage can.
Movement Into and Out of Ice
Similar processes occur when water molecules move between ice (the solid state) and liquid and gaseous states. When ice and liquid water are in contact, the energy levels of some molecules in the ice will overlap with some of those in the liquid. Some molecules will move from ice to water (melting) and others will move in the opposite direction (freezing). Likewise, when ice and vapor are in contact, some molecules in the vapor become solid ice (deposition), whereas some molecules in the ice move into the vapor (sublimation). How many move in each direction, and the resulting gains and losses of mass, depend on the overall energy level of the system. If there is equilibrium, the same number of molecules will move in opposite directions, but generally the ice, liquid, or vapor is losing mass to one of the other states.