What Are Heat and Temperature?
THE TERMS HEAT AND TEMPERATURE are used every day, but what do they actually mean? Temperature is a measure of the object's internal kinetic energy — the energy contained within molecules that are moving, and heat is thermal energy transferred from one object to another. Moving molecules drive many processes in the Earthocean-atmosphere system, such as evaporation, precipitation, and erosion.
What Is Sensible Heat?
1. The term heat is used in two ways. Scientists use heat to refer to the transfer of thermal energy from a warmer object to a cooler one or to the energy that is transferred in this way. The amount of heat is specified in a unit called a Joule, a measure of work or energy. Two common examples illustrate heat nicely.
2. What happens when you hold a cup of hot tea? Your hand feels heat coming from the cup. You are feeling the transfer of thermal energy from the cup to your hand. For this to happen, water molecules were heated and made to move. Once in the cup, the moving molecules collided with the inside of the cup, warming it. Then, that heat is transferred through the cup and against your hand via conduction — from the burner to the bottom of the kettle, to the water, to the cup, and finally to your hand. Conduction and convection both help distribute heat within the kettle and the cup.

3. What do you think happens when you hold a cold glass of ice water? In this case, the molecules in your hand are more energetic than the ones in the cold drink, so heat is transferred from your hand to the glass. Your hand feels cold because you are losing thermal energy to the cold glass.
4. This type of heat, which changes the temperature of two objects through exchange, is called sensible heat because we can sense it. But do we sense the actual temperature of an object or just the heat gain or loss? Try this experiment: Find a metal object and a wooden or plastic object in the same place. Place your hand on each and observe what you feel. Go do it, and then come back and continue reading. There, you no doubt sensed that the metal felt colder than the wood or plastic, but both have been in the room for a while and so are exactly the same temperature. This experiment shows that we sense heat gain and loss more than the actual temperature. In your experiment, metal conducted heat away from your hand faster, and so felt colder, but it wasn't.
What Is Temperature and How Do We Measure It?
Temperature is a quantitative measure of the average kinetic energy of molecules in an object — in other words, the hotness or coldness. Measurement of temperature of an object, whether a solid, liquid, or gas, involves the transfer of sensible heat from the object to some type of measuring device, usually a thermometer. Official temperatures are measured in a variety of ways, depending on the accuracy that is required and the location of the measurement.
The mercury-in-glass thermometer is the most familiar tool to measure temperature. Mercury is a convenient element because many of its physical properties remain consistent over the range of temperatures experienced on Earth. As the mercury's temperature increases, it expands and fills more of the tube. When it cools, the mercury contracts and withdraws down the tube.

Infrared thermometers calculate the temperature of a solid or liquid surface by pointing the sensor at the surface as it measures a range of wavelengths of energy emitted by that surface. Equations then relate wavelength to energy and energy to temperature. Infrared thermometry is a form of remote sensing, convenient when the surface is too far away, or too dangerous, to be measured directly.

When we need to measure sudden and slight temperature changes very precisely, we use special thermometers in which differences in energy content cause a thermoelectric response that can be wired to a computerized data recorder. The temperature can be calculated using specific equations that derive the amount of heat as a function of the amount of electrical current and the resistance of the electrical circuit.

Temperatures in the atmosphere are usually measured by weather balloons. These include instrument packages called radiosondes that measure a range of variables at various heights as the balloon ascends. Temperature is measured using thermoelectric principles. Wind, humidity, and other variables are also measured and relayed to the ground via radio signal.

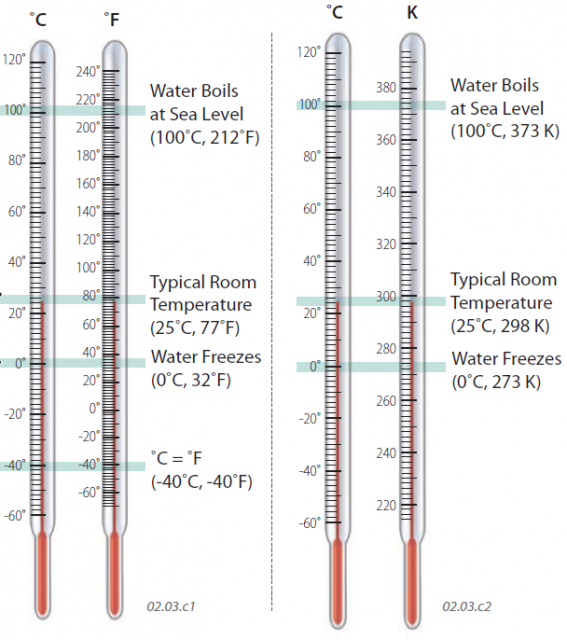
How Does the Fahrenheit Scale Relate to the Celsius and Kelvin Scales?
Most Americans are familiar with the Fahrenheit temperature scale, in which 32° represents the freezing point of water and 70° is a comfortable temperature. Nearly all nations except the U.S. use the Celsius scale, and scientists use the Celsius scale or a related scale called the Kelvin scale. We typically compare the scales with reference to the temperatures at which water freezes or boils, called the freezing point and boiling point, respectively.
In the Fahrenheit scale (°F), the boiling point of water is 212° (at sea level, but lower at higher elevations). In the Celsius scale (°C), the boiling point is 100° (at sea level).
Typical room temperature is 77°F, which is equivalent to 25°C.
The freezing point of pure water is 32°F, which is equivalent to 0°C. The Celsius scale was calibrated to the freezing and boiling points of water, with 100°C separating the two, a convenient scale.
The two scales correspond to one another at ?40° (that is, ?40°F equals ?40°C).
Between the boiling and freezing points of water, there are 100 Celsius degrees but 180 Fahrenheit degrees. So one Fahrenheit degree is only 100/180 (or 5/9) of a Celsius degree. This fact, along with the different “starting point” (i.e., the freezing point of water) forms the basis of converting between Fahrenheit and Celsius. To convert from Fahrenheit to Celsius, we must first subtract 32 degrees from the Fahrenheit temperature to allow for the fact that the starting point is offset by 32 degrees in the two systems. Then we must multiply by 5/9 to allow for the differences in the value of a degree on each scale. The equations for converting back and forth are as follows:
C = 5/9 x (F ? 32) F = (C x 9/5) + 32
Both the Celsius and the Fahrenheit scales are “arbitrary” in the sense that zero degrees doesn't mean that there is a lack of internal energy. Likewise, a doubling of the Fahrenheit temperature from 40° to 80° does not mean that there is twice as much internal energy. In scientific calculations, we need a temperature scale that allows us to relate changes in internal energy to the absolute amount of heat gained or lost by a system.
The Kelvin temperature scale (K) was devised as an “absolute” temperature scale to remedy these problems. In the Kelvin system, 0 K corresponds to the temperature at which no internal energy exists and all molecular motion theoretically ceases. This temperature is known as absolute zero, and is ?273°C or ?460°F. Doubling the internal energy of molecules would double their speed and be associated with a doubling of the Kelvin temperature. In the Kelvin system, water freezes at 273 K and boils at 373 K. Converting from Celsius to Kelvin temperature is easy:
K = C + 273 or C = K ? 273
Conversions between Fahrenheit and Kelvin can be made by converting first to Celsius and then to the other scale. Note that we do not use a degree symbol in reporting temperatures with the Kelvin scale.
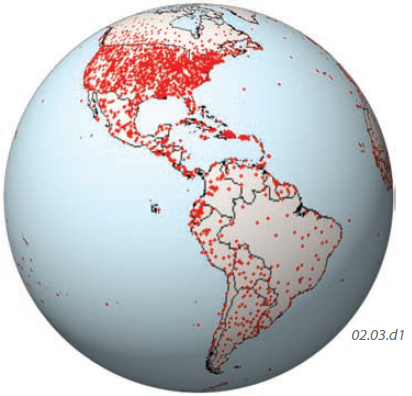
How Many Stations Report Temperature?
This globe shows part of the worldwide distribution of weather stations that have temperature data sets for at least many decades. The distribution is uneven, with most stations being on land, with relatively few stations in oceanic areas (on islands). Most stations are concentrated in densely populated areas, especially in the lowlands of more developed regions, such as the eastern U.S. Other regions, such as the center of South America (the Amazon rain forest), have very few stations to represent rather large areas. In recent decades, remote-sensing techniques have allowed truly global temperature coverage, but these data sets are not available as far back in time.