How Does Insolation Interact with the Atmosphere?
INSOLATION REACHES THE EARTH but has to pass through the atmosphere before it reaches us. The atmosphere does not transmit all of the Sun's energy; some wavelengths of energy are partially or completely blocked by atmospheric components, such as gas molecules. The interactions between insolation and the atmosphere explain many aspects of our world, like blue skies, red sunsets, and even the existence of life.
What Are the Principal Components of the Atmosphere?
The atmosphere is composed of gas molecules, especially nitrogen and oxygen, and small solid particles, including dust, and drops of water and other liquids. These particles and drops are together called aerosols, to convey that they are suspended in the atmosphere, floating with the moving air. Most gases and aerosols are produced by natural processes, but some are introduced into the atmosphere by activities of humans.
Gases — The atmosphere is more than 99% nitrogen (N2) and oxygen (O2), with lesser amounts of argon (Ar), and water vapor (H2O), as in the steam shown above. It contains trace amounts of other molecules, such as carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and sulfur dioxide (SO2).
Solid Particles — If tiny enough, solid particles can be suspended in the atmosphere. Such particles include volcanic ash, and wind-blown dust, salt, and pollen. They also include soot and smoke from natural and human-caused fires. Some small particles are produced by chemical reactions in the air.
Drops of Liquid—The atmosphere contains drops of water, with much smaller amounts of other liquids. Most water drops are tiny enough to remain suspended in the air as in clouds. If small drops combine or other-wise grow, they may become too heavy to remain suspended, falling as rain.
How Do Atmospheric Components Affect Insolation?
Insolation, like all types of electromagnetic radiation (EMR), can be affected by material through which it passes. As solar energy attempts to pass through the atmosphere, it interacts in various ways with the different atmospheric components. The types of interaction that occur depend on the size and physical nature of the component (e.g., solid versus a gas), the wavelength of the energy (blue versus red light, for example), and other factors.
Reflection and Absorption
Reflection — Some atmospheric components can partially reflect incoming insolation, such as by this snowflake. Reflected energy can be returned directly into space or can interact with other atmospheric components and remain in the atmosphere.
Absorption—An atmospheric component, such as this gas molecule, can instead absorb the energy, converting the incoming electromagnetic energy into kinetic energy expressed as motions of the molecule.

Scattering
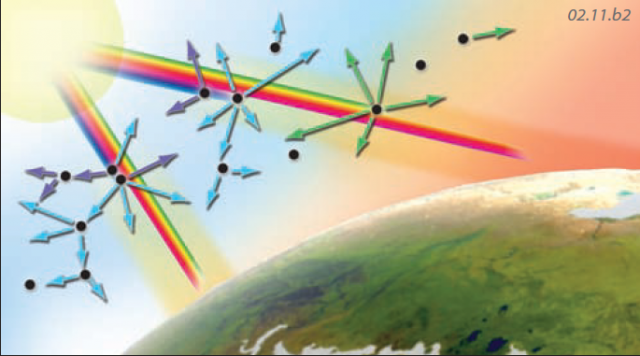
Scattering — Insolation can be scattered by atmospheric components, which send the energy off in various directions. Some processes of scattering affect shorter wavelengths of EMR more than longer ones; for example blue light is scattered more than red light. Other processes affect all wavelengths of solar energy equally.
Sky Color — As insolation enters the atmosphere, blue and violet light are preferentially scattered, and this scattered light causes us to see the sky as blue. The remaining light that passes through gives the Sun a yellowish color. When sunlight passes through the atmosphere at a low angle, as during sunrise and sunset, most colors, except orange and red, have been scattered out. Scattering of the remaining orange and red light produces the familiar orange and red glow.
How Do Different Layers of the Atmosphere Interact with Insolation?
If the atmosphere consisted of gases that did not interact with insolation, we would expect that its temperature would decline with distance from Earth. However, atmospheric temperatures exhibit some surprising changes with altitude, as a direct result of the interactions between insolation and atmospheric components, specifically gases.
1. Observations from high-flying planes, balloons, and rockets reveal that the atmosphere is divided vertically into four layers distinguished by their thermal properties. These layers are, from bottom to top, the troposphere, stratosphere, mesosphere, and thermosphere. In the troposphere and mesosphere, temperatures decline with increasing altitude, as expected, displaying a normal temperature gradient. Temperatures in the stratosphere and thermosphere, however, actually increase with altitude — a temperature inversion (reverse gradient). Why?
2. The unexpected change in temperature gradient in some layers is due to the fact that insolation arrives at the outside of the atmosphere in a variety of wavelengths of the electromagnetic (EM) spectrum, from very short ones (X-rays) to very long ones (radio waves). Most energy is in visible-light wavelengths, with significant amounts of adjacent wavelengths of ultraviolet (UV) and infrared (IR) energy. Some wavelengths of energy interact with certain atmospheric molecules, transferring energy as they do so. Different interactions occur in different layers, accounting for the differences in temperature gradient, and therefore the distinctions among the different layers. The four atmospheric layers are separated by distinct breaks called pauses.
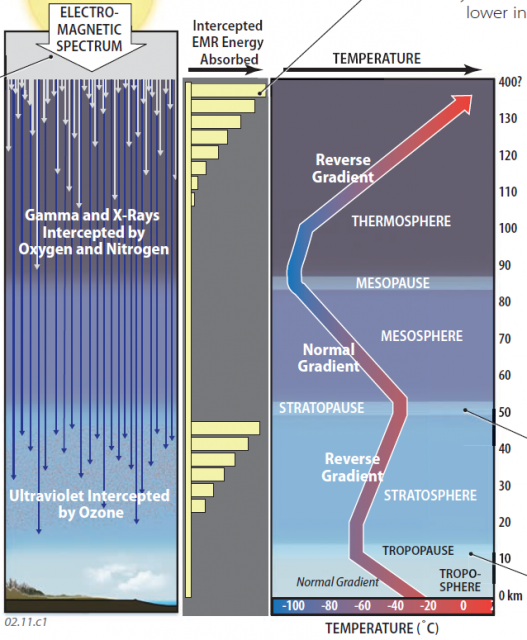
3. The very shortest and most energetic of EM radiation from the Sun are X-rays and gamma rays, with wavelengths approaching the size of gas molecules. These incoming wavelengths of EMR are effectively intercepted by the few molecules of nitrogen (N2) and oxygen (O2) in the uppermost parts of the atmosphere — the thermosphere. The greatest number of interceptions (and transfer of energy) occurs at the first opportunity for the gamma and X-rays to encounter these gases, at the outermost parts of the thermosphere. This causes the outer thermosphere to warm up, but so few molecules exist at such levels that you would freeze to death instantly, even at temperatures approaching 1,200°C. A progressively smaller proportion of these energetic rays penetrates lower in the thermosphere, so fewer energy exchanges occur, and temperatures decline downward across the thermosphere.
4. The next layer down, the mesosphere, possesses no particular properties to intercept wavelengths of insolation, so it displays a normal temperature gradient (temperature decreases upward). The boundary at the top of the mesosphere is the mesopause.
5. The stratosphere has relatively high concentrations of the trace gas ozone (O3), which effectively absorbs UV wavelengths. The same principle prevails as in the thermosphere, with the greatest amount of absorption occurring near the top of the stratosphere, which is therefore relatively warm. Progressively less absorption of incoming UV occurs downward, resulting in a decrease in temperatures downward — a temperature inversion. The top of the stratosphere is the stratopause.
6. Conditions in the troposphere can be very complex with the presence of various sublayers of air and the influence of clouds, but temperatures usually decrease upward (a normal temperature gradient). The top of the troposphere is the tropopause.
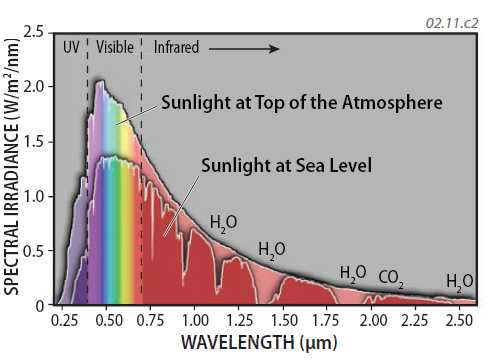
7. The greatest amounts of the Sun's emitted energy is at wavelengths of visible light, along with UV and IR. ( ) Interactions between solar energy and various components of the atmosphere (such as N2) cause some wavelengths of energy to be intercepted through absorption, scattering, or reflection in the atmosphere. Therefore, the spectrum of EM energy that reaches the surface is different in detail from that which enters the top of the atmosphere, as depicted by the curve showing the amount of insolation at sea level by this graph. Certain wavelengths of EMR are absorbed by certain components, such as water (H2O), oxygen (O2), and carbon dioxide (CO2), as expressed by dips in the curve for insolation received at sea level.