What Causes Water to Rise or Sink?
MOVEMENT WITHIN THE OCEANS arises from other factors in addition to shearing induced by the wind. Such factors include variations in the density of water in response to changes in its temperature and salinity. Changes in temperature and salinity occur from flows of fresh water into and out of the oceans as a result of precipitation, evaporation, ice formation and melting, and inputs from rivers. The temperatures of ocean waters and the relative amounts of fresh water control the density of water, and these differences in density can alter circulation of ocean waters.
How Does the Temperature of Water Control Its Density?
Like most substances, water changes its density when subjected to increases or decreases in temperature. Unlike other substances, however, water exhibits some peculiar density changes as it approaches freezing and then begins freezing.
Polarity of the Water Molecule
1. A key attribute of water that controls its behavior is its polarity. Recall that in a water molecule, the two hydrogen atoms are on one side of the oxygen, giving the molecule a polarity, which causes water molecules to be attracted to charged atoms (ions). This allows water to interact with other chemical substances, such as salt.
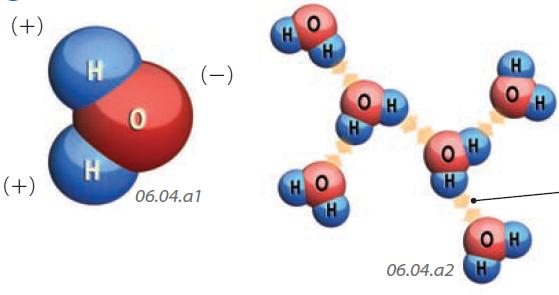
Hydrogen Bonding
2. Water molecules are attracted to each other as well as to ions. In water, a bond called a hydrogen bond forms between one molecule's hydrogen atom and another molecule's oxygen atom.
3. Hydrogen bonds form when the polarities of two adjacent water molecules attract one another, causing a bond to form between the molecules. The hydrogen bond is responsible for some of water's unique properties (e.g., viscosity and surface tension).
Density of Water as a Function of Temperature
4. This graph shows how the density of water changes with temperature. The curve on the right side shows how liquid water changes density in temperatures slightly above freezing. The vertical break marks 0°C, the temperature at which water freezes. The angled line on the left shows how ice changes density with temperature. Examine the main features of this graph before we explore it further. Read clockwise around the graph, beginning on the other side.
5. At temperatures above 4°C, water, like most substances, becomes more dense as it cools, as shown by moving from right to left up the sloping line on the right side of the graph. With cooling temperature, individual molecules become less energetic and can occupy a smaller volume, thereby increasing the water's density.
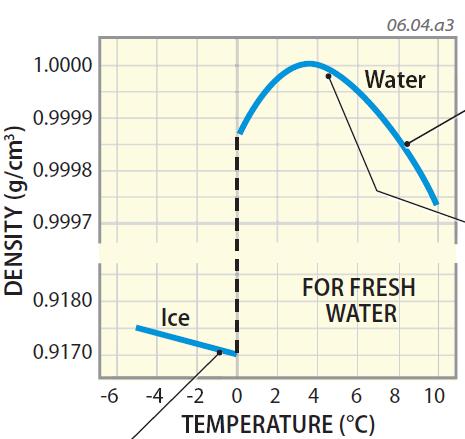
6. At a temperature of 4°C, however, water begins to decrease in density, as is shown by the downward bend in the graph between 4°C and 0°C. As a result, water reaches its maximum density (1 g/cm3) at a temperature of approximately 4°C (39°F). This decrease in density occurs because, at temperatures below 4°C, the strength of the hydrogen bonds starts to exceed the disruptive random motions of the less energetic molecules, and the molecules start to organize themselves into a more regular arrangement that occupies more volume and decreases density.
7. As ice (the solid state of water) forms, the crystalline structure of the hydrogen bonds dominates, placing adjacent molecules about 10% more distant, on average, from their nearest neighbors than when in the liquid form at 4°C. This causes the density of ice to drop to about 0.917 g/cm3. This decrease in density when water freezes causes ice to float on water —less dense materials, like wood and ice, float on a more dense liquid, like water. Our world would be a very different place if ice were more dense than water. Ice would begin to grow upward from the ocean floor over time until it occupied a very large percentage of the oceans.
Sinking and Rising of Water as a Function of Temperature
8. These density changes of water as a function of temperature have huge implications for our natural world, many of which will be discussed later in this chapter and throughout the book.
9. At temperatures between freezing and 4°C, warmer waters are more dense than cooler waters (if they have the same salinity), and will therefore sink, helping to redistribute warmth downward in the ocean. Once ice forms, it is even less dense than warmer waters, so it floats. In most substances, the solid state is more dense than the liquid one, but not for water. Ice is approximately 9% less dense than liquid water.
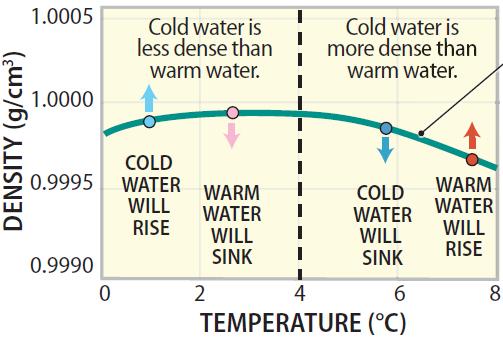
10. At temperatures above 4°C, water behaves like most other substances, becoming less dense as it warms. At temperatures above 4°C, cooler water is more dense than warmer water and will therefore sink.
How Does Change in Salinity Affect the Density of Water?
How Salt Occurs in Water and Changes Its Density
1. We all know that water can dissolve some solid materials, like salt, but how does water do this? When we add salt crystals, like the one in the center of this block, to a pot of water, each crystal is surrounded by water molecules. In the salt mineral halite, sodium atoms have loaned an electron and so have a positive charge (Na+). Such positive ions are called cations. Chlorine has gained an electron and so has a negative charge (Cl?). Such negative ions are anions.
2. The negatively charged chlorine anion is attracted to the positive (H) end of the water molecule. If this attraction is strong enough, it can pull the chlorine away from the halite crystal and into the water.
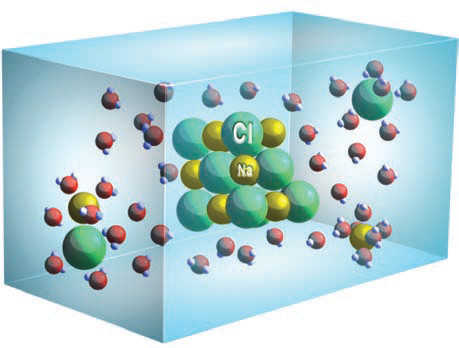
3. In a similar manner, sodium in halite is a positively charged cation (Na+), and thus is attracted to the negative side of any adjacent water molecule. This attraction can pull the sodium ion away from the halite crystal and into the water.
4. Once dissolved in water, the positively charged sodium cation (Na+) will be surrounded by the negative sides of water molecules, limiting its ability to rejoin the salt crystal. The negatively charged chlorine anion (Cl?) is likewise surrounded by the positive side of the encircling water molecules.
5. As the salinity of water increases so does its density, in a reasonably linear fashion. Elevated saline content in water increases its density, so it will sink. Less saline water will rise. The exact nature of the relationship between salinity and density also depends upon temperature.
Decreasing the Salinity of Water
6. One way to decrease the salinity of water is to dilute it with fresh water, such as by adding precipitation to the body of water.
7. Dilution of salt content also occurs from the introduction of fresh water, as from a stream. Most rivers contain dissolved salt, but much less than the sea.
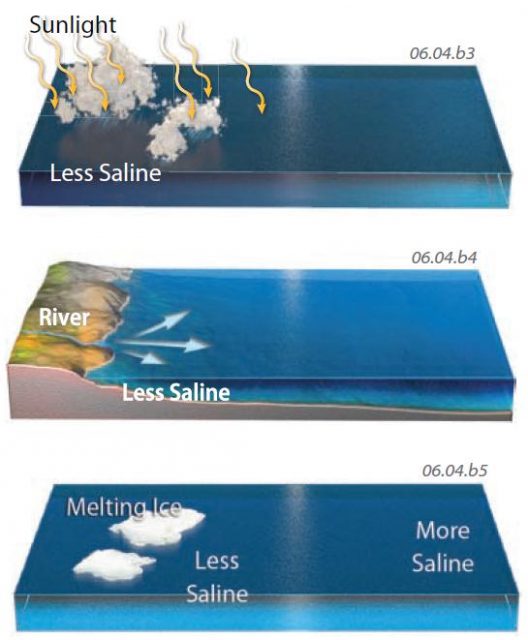
8. Ice consists of fresh water because when it freezes it does not easily incorporate salt into its crystalline structure. When ice melts, it adds fresh water to the body of water, diluting the salt content and decreasing the salinity. Ice melting on land likewise provides abundant fresh water to streams, most of which drain into some body of water.
9. Several types of ice occur in water. The most familiar type is an iceberg, which is a piece of ice broken off a glacier that flowed into a sea or lake. The second type is sea ice (not shown), which forms from freezing of seawater into a smoother layer of ice coating part of the sea.

Increasing the Salinity of Water
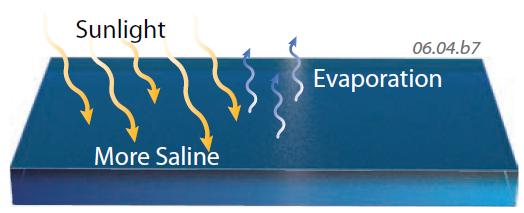
10. The most obvious way to increase the salinity of water is through evaporation, which removes water molecules but leaves the salt behind. The water vapor contains no salt and can be carried far away by motion in the atmosphere, eventually producing freshwater precipitation as rain, snow, sleet, or hail. This fresh water forms the streams and glaciers on land.
11. Salinity also increases when ice forms on the sea. Fresh water is removed during freezing, leaving behind the salt in the water. As a result, the salinity of seawater increases below and adjacent to sea ice. If the sea ice melts, as can occur during the summer, it introduces the fresh water back into the ocean.

- How Do Sea-Surface Temperatures Vary from Place to Place and Season to Season?
- What Is the Global Pattern of Surface Currents?
- What Causes Ocean Currents?