What Is Electromagnetic Radiation?
ELECTROMAGNETIC RADIATION is one of the fundamental forces of nature. It dominates our daily interactions with the world, determining the color of objects, the character of the air we breathe, and the physical characteristics of the water we drink. Electromagnetic radiation is essential to the operation of weather and our climate system and to all lifeforms on the Earth's surface, including us.
What Are Some Common Examples of Electromagnetic Radiation?
Electromagnetic radiation (EMR) is all around us, although we may not be aware of all of its manifestations. Its most obvious expression is as light, which is the only kind of EMR that we can observe with our eyes. EMR is also what causes sunburns, is the heat we feel from a heat lamp, and is expressed in all sorts of wireless communications, including TV, radio, and WiFi.
Visible light is one type of electromagnetic radiation (EMR). Visible light is itself composed of different types of EMR, which we can observe by using a prismThere are many types of EMR that are not visible to us. These include radio waves (like WiFi), X-rays, and microwaves that cook our food. These three types of EMR cannot be observed, but are used daily. to spread out visible light into its rainbow colors. On following pages, we explain what causes different colors.
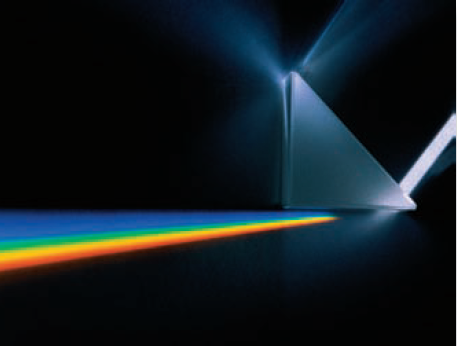
What Is the Character of Electromagnetic Radiation?
Electromagnetic radiation consists of energy radiated from charged particles and manifested as interacting electrical and magnetic fields. For most applications, it is useful to think of electromagnetic radiation as a series of waves that, if unconfined, radiate out in all directions. When observed in certain ways, such as at microscopic distances and exceptionally short timescales, EMR appears to be composed of particles. Here, we will treat EMR as waves.
1. To visualize the rather abstract concept of EMR, think of it as a series of waves of electrical and magnetic energy that are moving from one place to another, in this case, from left to right. Like any waves, some parts are higher and some are lower. The direction in which a wave is moving is the direction of propagation. The wave shown here is propagating from left to right. To envision how such a wave moves, think about what happens when you shake the end of a rope or string. The rope curves into the series of waves, which move from your hand outward to the end of the rope.
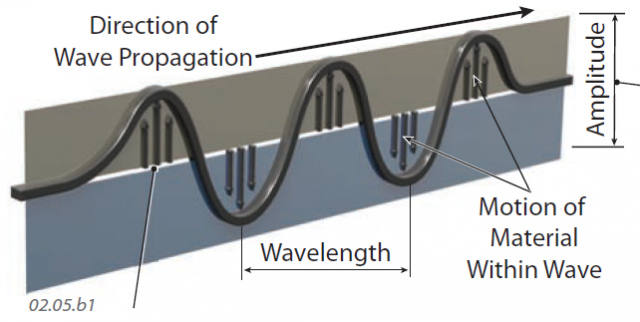
2. In this type of wave, the motion of any part of the wave is mostly up and down, parallel to the small arrows on this figure — the rope stays in your hand, so it is not moving away from you, although the waves are. Motion within the wave (up and down) is perpendicular to the direction of propagation of the wave (left to right).
3. In describing such waves, whether in a rope or EMR, the term amplitude refers to the height of the wave, from trough to crest. The greater the difference in height between the top and bottom of the wave, the greater the amplitude. Surfers like large amplitude waves.
4. We use wavelength to describe the distance between two adjacent crests (tops) or two adjacent troughs (bottoms). The longer the distance between two adjacent crests or troughs, the greater the wavelength. If we know the speed of the wave, we can describe the frequency — the number of waves per second passing a point.
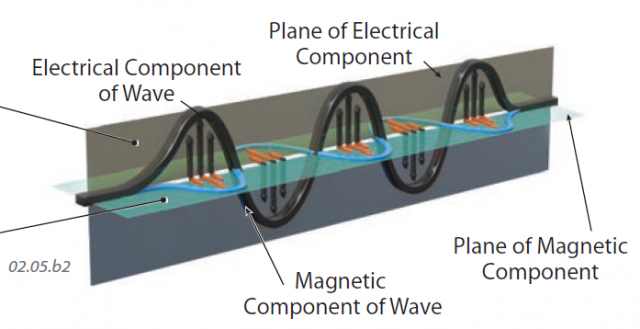
5. Electromagnetic waves travel through electric and magnetic fields, which can be envisioned as two mutually perpendicular planes. In this figure, the electric field, containing the electrical component of the wave, is vertical (dark horizontal (colored blue with the orange horizontal arrows).
6. The electrical and magnetic waves have the same wavelength, and the crests of the two types of waves are the same distance along the direction of wave propagation (i.e., the crests line up). Motion in both types of waves is perpendicular to the direction the wave propagates, as described in the previous figure. Electromagnetic waves get their name from these linked electrical and magnetic waves that move in unison.
How Is Electromagnetic Energy Generated and Transmitted?
Electromagnetic radiation is generated by changes associated with charged particles — atoms and molecules produced by vibrations within the particle, by changes in the energy level of electrons, and by fusion of particles, as occurs in the Sun. The Sun is the dominant source of energy for Earth, emitting EMR at a variety of wavelengths.
1. Atoms have a tiny central core — the nucleus — that is so much smaller than the entire atom that it cannot be shown here. The red and yellow spheres are electrons. Groups of electrons travel around the nucleus at different distances, called electron shells. Each shell has a different level of energy, increasing away from the nucleus. If an electron in an outer, higher energy shell drops into an inner, lower energy shell, it must emit the extra energy as an EMR wave, which radiates out in all directions (shown here as one direction for simplification).
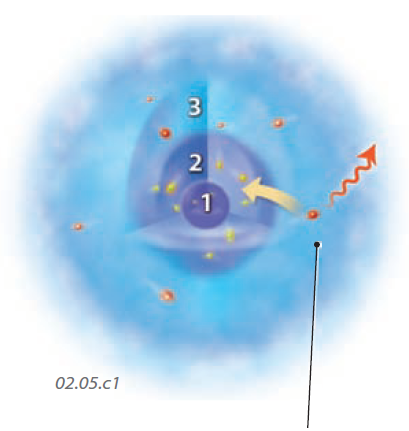
2. Electrons within atoms and the bonds (shown here as springs) within molecules vibrate back and forth, changing positions slightly. This motion emits EMR, and the faster such motions occur, the higher the frequency of the EMR that is emitted.
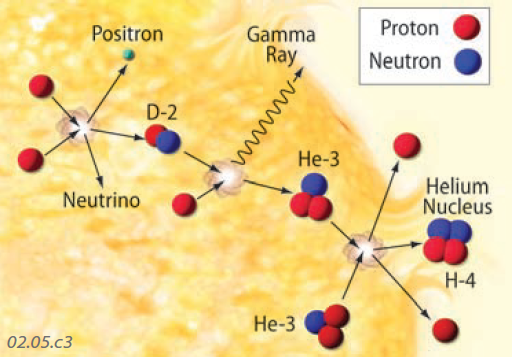
3. The Sun is huge and extremely hot. It is nearly all composed of hydrogen and helium, the two lightest chemical elements. Through a series of steps, protons and neutrons join together to produce larger particles, such as helium — the process of fusion, which releases huge amounts of EMR that radiate outward.
4. Earth is approximately 150 million km from the Sun, and the nearly complete vacuum of space separating the two objects eliminates the possibility of conduction, convection, and advection, all of which require a medium for the transfer to occur (there is no medium in a vacuum). The Sun's immense mass means that very little material can escape the Sun's gravitational pull. Therefore, almost all energy comes to Earth via radiation, which can pass through a vacuum, as illustrated by the fact that light from a flashlight can easily pass through sealed flasks, one containing air and other containing a vacuum.

How Much Energy Does an Object Emit?
Scientists have discovered a number of important quantitative relationships, some of which are considered laws of nature, including those that govern the production and transfer of energy. One important relationship is the Stefan-Boltzmann Law, which relates an object's temperature to the amount of EMR it emits.
1. The Stefan-Boltzmann Law is represented graphically by this figure, which plots the amount of emitted energy as a function of temperature in Kelvin (K), for a specific type of object that absorbs all the energy that strikes it. We are simplifying this discussion a bit, which your instructor will recognize, but we don't think you'll mind.
2. The graph starts at a temperature of zero Kelvin (?273°C), and at this temperature the amount of emitted energy is zero. All molecular motion ceases at zero Kelvin — which is why it is called absolute zero — so at this temperature no radiation would be emitted. No motion means no EMR.
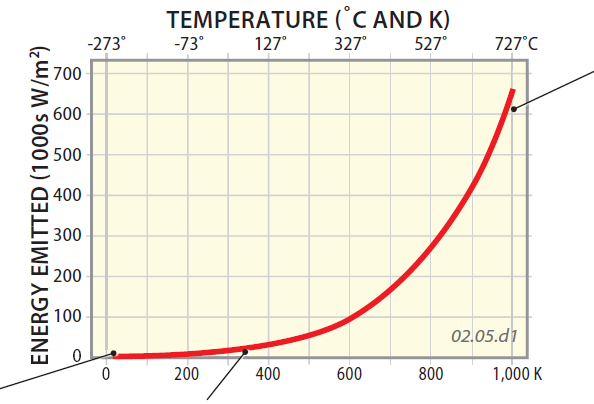
3. With an increase in temperature (moving to the right on the graph), the amount of energy emitted increases, slowly at first. What this indicates is that as an object increases in temperature, the object emits more energy (in the form of EMR). In other words, hot objects emit more radiation than do cold ones. Since temperature represents the amount of motion associated with the atoms and molecules within a material, the graph means that the amount of EMR increases as the amount of atomic scale motion increases.
4. At higher temperatures, the amount of energy emitted increases dramatically as a function of temperature, much faster than it did at lower temperatures. Compare how much the amount of emitted energy increased from 200 to 400 K with how much it increases from 800 to 1,000 K. The shape of the curve indicates that hot objects emit much larger quantities of energy than cooler ones.
5. Using the equation from which this graph was derived, and factoring in some other aspects not discussed here, we can use an object's surface temperature to predict how much energy it should emit. The Earth's average temperature is 283 K, whereas the Sun's average surface temperature is 6,000 K (way off the graph) — we would predict the Sun emits much more energy than the Earth. From the calculations, the Sun should emit more than 200,000 times more energy than Earth!