The Ozone Layer— Shield to Life
High above the Earth's surface lies an atmospheric layer rich in ozone—a form of oxygen in which three oxygen atoms are bonded together (O3). Ozone is a highly reactive gas that can be toxic to life and damaging to materials, but high in the atmosphere it serves an essential purpose—sheltering life on the Earth's surface from powerful ultraviolet radiation emitted by the Sun. Without the ozone layer to absorb this radiation, bacteria exposed at the Earth's surface would be destroyed, and unprotected animal tissues would be severely damaged. The ozone layer is presently under attack by air pollutant gases produced by human activity. The most important gases are chlorofluorocarbons, or CFCs—synthetic industrial chemical compounds containing chlorine, fluorine, and carbon atoms. Although CFCs were banned in aerosol sprays in the United States beginning in 1976, they are still used as cooling fluids in some refrigeration systems. When appliances containing CFCs leak or are discarded, their CFCs are released into the air. Ozone is constantly being formed and destroyed by chemical reactions in the upper atmosphere, and the balance between formation and destruction determines the concentration of ozone. CFC molecules move up to the ozone layer where they decompose to chlorine oxide (ClO), which attacks ozone, converting it to ordinary oxygen (O2 ) by a chain reaction. This lowers the concentration of ozone, and with less ozone, there is less absorption of ultraviolet radiation.
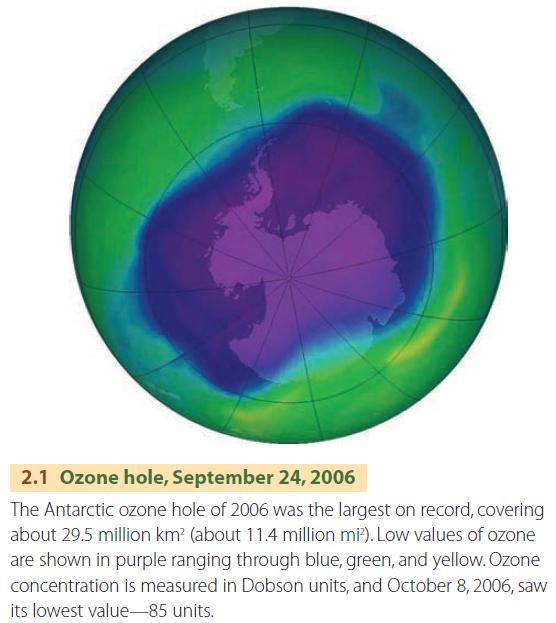
A “hole” in the ozone layer was discovered over the continent of Antarctica in the mid-1980s (Figure 2.1). In recent years, the ozone layer there has been found to thin during the early spring of the southern hemisphere, reaching a minimum during the month of September or October. Typically, the ozone hole slowly shrinks and ultimately disappears in early December.
In the northern hemisphere, conditions for the formation of an ozone hole are not as favorable. But arctic ozone holes have occurred several times in the past decade, with a strong arctic ozone hole observed in 2005. Atmospheric computer models have projected more such events in the period 2010–2019.
Aerosols inserted into the stratosphere by volcanic activity also can act to reduce ozone concentrations. The June 1991 eruption of Mount Pinatubo, in the Philippines, reduced global ozone in the stratosphere by 4 percent during the following year, with reductions over midlatitudes of up to 9 percent.
Since 1978, surface-level ultraviolet radiation has been increasing. Over most of North America, the increase has been about 4 percent per decade. This trend is expected to increase the number of skin cancer cases. Crop yields and some forms of aquatic life may also suffer. Today, we are all aware of the dangers of harmful ultraviolet rays to our skin and the importance of using sunscreen before going outdoors.
In response to the global threat of ozone depletion, 23 nations signed a treaty in 1987 to cut global CFC consumption by 50 percent by 1999. The treaty was effective, and by 1997, stratospheric chlorine concentrations had topped out and started to fall. In 2003, scientists using three NASA satellite instruments and three international ground stations confirmed a slowing in the rate of ozone depletion starting in 1997.
In operation for two decades, the international agreements have had an effect. Though not a reversal of ozone loss, the trend is encouraging. Current predictions show that the ozone layer will be restored by the middle of the century.