What Is Energy and How Is It Transmitted?
THE TRANSMISSION OF ENERGY, and the interactions between energy and matter, define the character of our planet and control weather, climate, and the distribution of life, including humans. Here, we examine the fundamentals of energy, including what it is, where it comes from, and how it is moved from one place to another.
What Is Energy?
All matter contains energy, which is the capability of an object to do work, such as pushing or pulling adjacent objects, changing an object's temperature, or changing the state of an object, as from a liquid to a gas. How is such energy expressed at the scale of atoms and molecules (combinations of atoms)?
1. Energy is expressed at an atomic level by motions of atoms, molecules, and their constituent parts. These motions include changes in position and in-place vibrations and rotations. The more motion the atoms and molecules display, the more energy the system contains. Atoms and molecules in a gas can move at over 200 m/s, or about 500 miles/hour. These fast-moving objects collide with adjacent ones, causing atmospheric pressure in a similar fashion to the way in which air molecules hold out the walls of an inflated balloon.
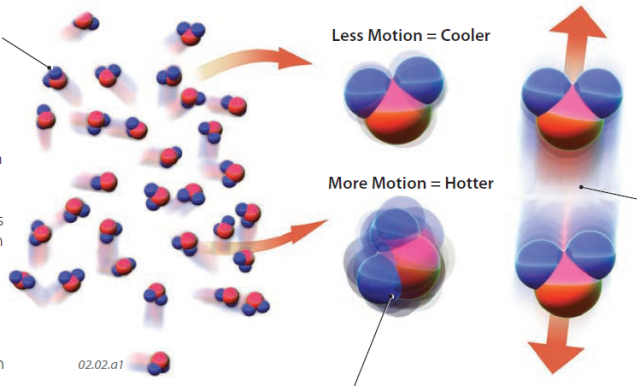
2. The temperature of an object is a measure of the average energy level (motions) of its molecules and atoms. The molecules of all objects on Earth, regardless of temperature, are moving, some more than others. This type of energy, due to motions of objects, is called kinetic energy.
3. Energy can also be tied up within the atomic or molecular structure of matter. When we heat a liquid, we impart energy to the constituent molecules, causing some of the molecules to move apart, escaping as vapor. But these molecules also carry stored energy, which can be released when the molecules recombine into a liquid. This type of energy is called potential energy, because it is not being expressed directly, but could potentially be released. Where potential energy is related to a change in the state of matter, such as from a liquid to a gas, as in the example presented here, it is called latent energy (latent means hidden).
How Does Energy Relate to the State of Matter?
In our everyday world, matter exists in three different forms or states — solid, liquid, and gas. The energy content of the material determines which of these states of matter dominates at any time and place.
1. When matter is in a solid state, the constituent atoms and molecules are bound together, such as in the structured internal architecture of a crystal. The energy levels (motions) of the atoms and molecules are low enough that the solid can withstand the vibrations and other motions without coming apart. As a solid is heated up by an external energy source, like the Sun, the motions become more intense.
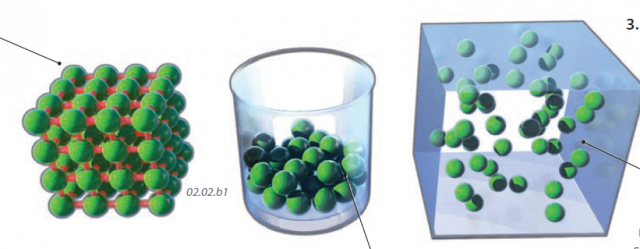
2. If enough energy is added to a solid, the motions begin to break the bonds that hold the solid together. The material begins to melt, turning into a liquid, which is a collection of mobile atoms and molecules that more or less stay together but are not held into the rigid form of a solid. For example, energy provided by the environment increases the vibrations of the water molecules in an ice crystal until the bonds holding the ice crystal together begin to disintegrate and the ice melts.
3. If even more energy is added, such as by placing the liquid water on a hot burner, the added energy causes even more energetic motions of water molecules in the liquid, allowing more and more molecules to break free of the liquid and enter the air as a gas — water vapor. As a result, atoms and molecules in their gaseous state are more energetic than in their solid or liquid equivalents. Not only are the molecules in a gas intensely vibrating and rotating, molecules can now move freely through the air at high speeds — having a large amount of kinetic energy.
What Occurs During the Processes of Warming and Cooling?
When something changes temperature, we say that it is warming or cooling, but what actually occurs during a change in temperature? For warming or cooling to occur, energy must be transferred from one object to another, such as from a flame to the air around it.
When an object increases in temperature, it is warming. Warming occurs where an object gains energy from the surroundings, resulting in the increase in temperature. You gain energy when you feel the warming benefit of a campfire. There is no need to touch the flames to feel the warming effect. Energy is transmitted from the fire to the surroundings. When you heat up water in a pan on the stove, energy is transferred from the burner to the pan and then from the pan to the water. If you add enough energy, the water molecules become so warm and energetic that they start to escape the pan during the process of boiling.

The opposite of warming is cooling. Cooling is the loss of energy to the surroundings. When you are out in cold weather, you lose energy to the cold air, causing you to feel cold. A similar process occurs when you add warm water to ice cubes in a glass dish. Initially, the water is warmer than the ice, but once the water and ice are in contact, molecular motion in the water is transmitted to the cooler ice. This transfer of energy adds more energy to the ice, eventually melting it. As the surrounding water loses energy to the ice, it cools, resulting in cold water. Cooling indicates a loss of energy, whereas warming indicates a gain of energy.
What Are the Four Dominant Types of Energy Transfer?
Heat is thermal energy transferred from higher-temperature to lower-temperature objects. Heat transfer, also called heat flux or heat flow, results when two adjacent masses have different temperatures. The four mechanisms of heat transfer are conduction, radiation, convection, and advection. An understanding of the various mechanisms of energy transfer is crucial in understanding weather, climate, evolution of landscapes, and many of our daily activities.
Conduction — A water-filled pan on a burner gets hot as thermal energy is transferred by direct contact between the burner and an, and the pan and water. Heat transfer by direct contact is conduction, which involves transferring thermal energy from the warmer object (with more energy) to the cooler one (with less energy). Energy can only be transferred from the more energetic one to the less energetic ones. Molecules are most densely packed in solids and least densely packed in gases, so conduction is a more important mode of transfer in solids, whereas gases conduct energy much less efficiently.
Radiation — A hot burner on a stove can warm your hands a short distance away. Such warming occurs because heat from the burner radiates through the air, a process called radiation or radiant heat transfer. Radiation is energy transmission by means of electrical and magnetic fields. All objects constantly emit various kinds and quantities of this particular type of electrical and magnetic radiation, which is known as electromagnetic radiation. This form of energy is even capable of passing through a vacuum.
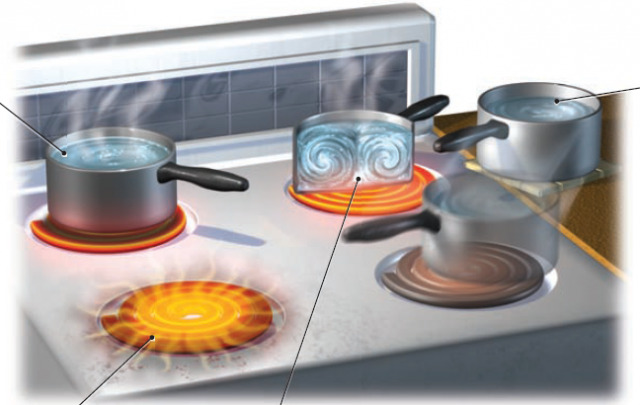
Convection — Energy is conducted through the base of the pot and into the lowest layer of water contacting the pot. These molecules move more, requiring more room to do so, which causes the volume occupied by the warmer water to increase and its density (mass per unit volume) to decrease. Being less dense than the overlying cooler water, the warm water rises. When the rising water reaches the surface, it cools and flows back down the sides. This type of vertical heat transfer by flow of a gas, a liquid, or a weak solid is convection. If the material flows around a circular
Advection — Moving a pan full of hot water away from the stove also transfers heat from one place to another. Energy transfer by the horizontal movement of a material, such as moving the pan sideways off the burner, is called advection. Fog along many coastlines results from warm air flowing sideways and mixing with cooler air over the coast. This horizontal transfer of energy via the moving air is advection.