What Is Ozone and Why Is It So Important?
OZONE IS AN ESSENTIAL GAS in the atmosphere, shielding life on the surface from deadly doses of ultraviolet radiation from the Sun. In the past several decades, there has been major concern about the loss of ozone in our atmosphere, particularly a seasonal decrease in ozone above Antarctica. How can a gas that generally constitutes less than one molecule in every 10 million in the air be so important, and why does the Antarctic region experience severe ozone loss?
What Is Ozone and Where Does It Occur?
Ozone is a molecule composed of three oxygen atoms bonded together (O3), instead of the much more common arrangement of two oxygen atoms in a molecule of oxygen gas (O2). More than 90% of ozone occurs in the stratosphere, but ozone also occurs in lesser amounts in the troposphere and mesosphere.
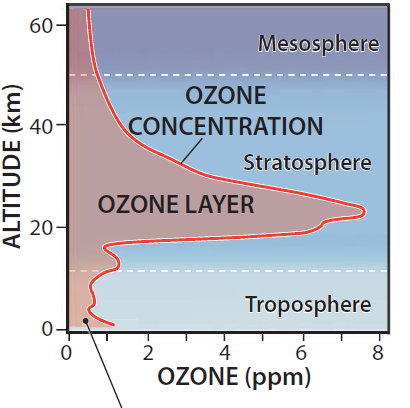
This graph plots the concentration of ozone in the atmosphere as a function of altitude above Earth's surface. Note that concentrations are very low overall, measured in parts per million (ppm — the number of ozone molecules for every one million molecules of atmosphere). The maximum concentration is in the middle of the stratosphere, a zone called the ozone layer, but even here the ozone concentration is only 6 to 8 ppm. Ozone concentrations decrease rapidly into the overlying mesosphere.
There is a slight increase in concentrations low in the troposphere, just above the surface. Whereas ozone in the ozone layer (stratospheric ozone) is beneficial to life, ozone near the surface (ground-level ozone) is a harmful air pollutant, produced by sunlight striking hydrocarbons, such as car exhaust, in the air.

Ozone is a component of smog, a type of air pollution common in larger cities, especially under certain atmospheric conditions. The term smog was derived from air pollution that looks like smoke and fog.

Ozone is also produced naturally due to ionization of oxygen gas molecules in the air near lightning. In such settings, we can sense ozone's distinctive smell, and this is the origin of the term ozone, after the Greek verb for “to smell.”
How Is Ozone Produced and Destroyed in the Stratosphere?
1. Electromagnetic radiation (EMR) with a wavelength of visible light or shorter (e.g., ultraviolet) has the ability to affect the chemical bonds in a compound, a process called photodissociation. Such processes occur throughout the atmosphere, but particularly in the stratosphere, where (1) much of the EMR arriving from the Sun has not yet been absorbed and (2) the concentration of molecules becomes high enough to allow photodissociation
to proceed. This figure shows how photodissociation forms and destroys ozone.
2. Oxygen in the atmosphere is mostly oxygen — gas (02), composed of two oxygen atoms bonded together. These molecules absorb EMR from the Sun, including several types of ultraviolet (UV) energy. They are strongly affected by an energetic type of UV called UV-C, which has relatively short wavelengths. UV-C can break the two oxygen atoms, liberating a free (unbonded) oxygen atom (O).
3. The freed oxygen atom can quickly bond with another free oxygen atom, forming a new molecule of oxygen gas.
4. Alternatively, a freed oxygen atom can combine with an existing oxygen gas molecule to form a molecule of ozone (03). This process is the way ozone is produced in the atmosphere.
5. Ozone molecules are capable of absorbing a longer wavelength form of ultraviolet called UV-B, in addition to UV-C. UV-B has lower energy than UV-C, but more of it reaches Earth's surface. UV-B is the wavelength of ultraviolet radiation that causes sunburn and contributes to skin cancer. It also produces vitamin D in our bodies when it interacts with our skin.
6. UV-C and UV-B can photodissociate an ozone molecule, leaving a molecule of oxygen gas and a free oxygen atom. These can recombine with other atoms and molecules to form another molecule of ozone, so the process of formation and destruction of ozone is a cycle, with ozone and oxygen gas continuously being broken part, combined, and broken apart again. If the rates of formation and destruction of ozone molecules are equal, the ozone concentration will remain constant. Variations in atmospheric conditions, however, change the relative rates, causing concentrations to change over time and from place to place.
What Is the Distribution of Ozone in the Atmosphere?
These two globes show the amount of ozone in the atmosphere at two different months, as measured from a satellite. In general, ozone concentrations are relatively low (green) over the equator, and are higher (yellows and oranges) in middle and high latitudes. The difference between the two globes shows that ozone concentrations change with the season, responding to changes in the patterns of insolation. Ozone amounts are expressed as Dobson units (DU).
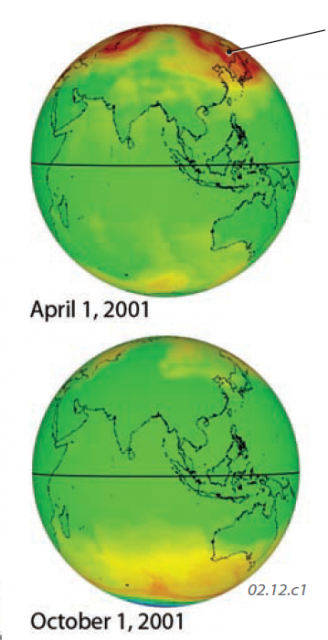
In April, the highest concentrations are in higher latitude regions of the Northern Hemisphere. In October, the highest values are in high latitudes of the Southern Hemisphere, but there is a huge area with very low concentrations centered over the South Pole (at the bottom of the globe). This low exists because total darkness during the Antarctic winter lasts for several months, during which time there is no insolation to form ozone, but ozone continues to be chemically destroyed (see below).
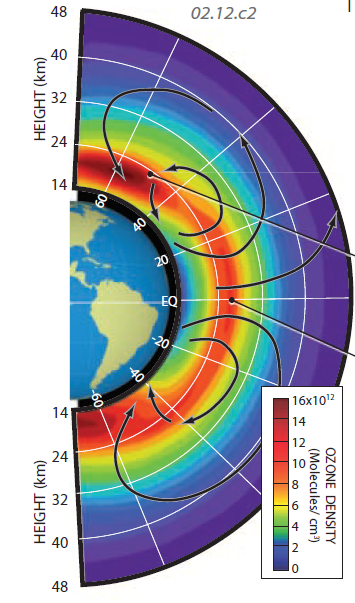
This figure shows ozone concentrations in the main part of the stratosphere. Ozone is most concentrated (red zones on the figure) in the lower middle stratosphere — the ozone layer. The highest concentrations are in high latitudes, and below an altitude of about 25 km. In equatorial regions, the zone of maximum ozone is at higher altitudes, typically more than 25 km. Production of ozone is greatest in the tropical equatorial regions, which receive the most insolation, including UV, but a very slow-moving circulation pattern in the stratosphere moves the ozone upward and laterally toward the poles, accounting for the higher concentrations there.
What Is Causing Depletion of Ozone and Formation of the “Ozone Hole”?
Ozone is critical to life because it shields us from dangerous UV-C and UV-B radiation. In the past half century, we became aware that our protective ozone shield was being depleted by human activities, especially the production of chlorofluorocarbons (CFC), chemicals released from aerosol cans, air conditioning units, refrigerators, and polystyrene. As a result, production of CFCs was limited by international agreement, the Montreal Protocol, adopted in the 1990s.
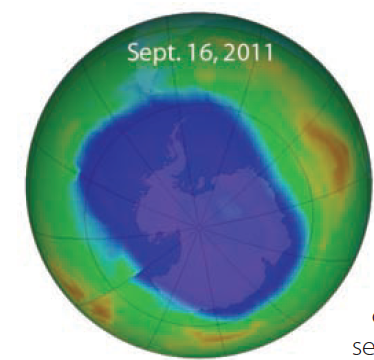
1. This image shows ozone concentrations in the Southern Hemisphere as measured by a NASA satellite, with the lowest amounts in purple. The image, taken in September 2011, shows 10.6 million square miles of the Antarctic region with severe ozone depletion. This depletion is described as “thinning of the ozone layer.”
2. Most ozone is destroyed by natural processes, but humans have introduced chemicals that accelerate such losses. Chlorofluorocarbons (CFCs) contain halogens, elements like chlorine and bromine that easily bond with another element or molecule. Halogens can break apart ozone by attracting one of the oxygen atoms away, forming a new molecule, such as one with chlorine and oxygen (ClO).
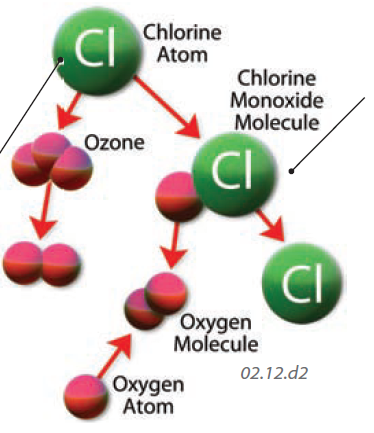
3. The ClO molecule is short lived, and the chlorine atom breaks away to combine with other O atoms, typically outcompeting O2 for bonding with O, thereby breaking apart ozone or keeping it from forming. On average, a single Cl may be responsible for the destruction of up to 10,000 ozone molecules. More than 70% of halogens in the atmosphere were introduced by humans.
4. Halogens are regarded as a major factor in the depletion of the ozone layer in the Southern Hemisphere, shown in the top globe. The bottom globe shows the Northern Hemisphere at an equivalent time of year (spring in both places). Why is there thinning of the ozone layer over Antarctica but less so over the North Pole?
5. The contrasting geography of the two polar regions plays a major role. Antarctica, in the center of the top globe, is a continent surrounded by ocean. This arrangement produces a zone of rapid circumpolar winds, which effectively exclude the import of ozone from nonpolar areas.
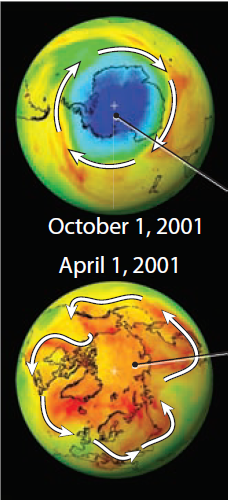
6. In contrast, the Arctic is an ocean surrounded by irregularly shaped continents (Eurasia and North America). The alternating pattern of oceans and continents induces far greater north-south movement and complex wind patterns, which encourage the exchange and mixing of gases, including ozone, into and out of the northern polar atmosphere.