What Happens to Insolation That Reaches the Surface?
APPROXIMATELY HALF OF INSOLATION is transmitted to Earth's surface, and this energy is variably reflected, absorbed, and re-emitted. Earth absorbs energy of short wavelengths, including insolation, but re-emits it at longer wavelengths. Certain greenhouse gases in the Earth's atmosphere interact with this outgoing long-wavelength radiation, complicating the return of this energy to space and helping keep our planet at a hospitable temperature.
How Does Insolation Interact with the Surface?
Insolation that reaches Earth's surface is at short wavelengths, centered on the visible spectrum. It also includes ultraviolet (UV) and infrared (IR) wavelengths adjacent to the visible spectrum, commonly called near-UV and near-IR, respectively. Such electromagnetic radiation (EMR), with wavelengths less than 4 mm, is called shortwave radiation. By contrast, Earth emits energy at longer wavelengths, or longwave radiation. Shortwave radiation that reaches Earth's surface can be converted into other forms of energy.
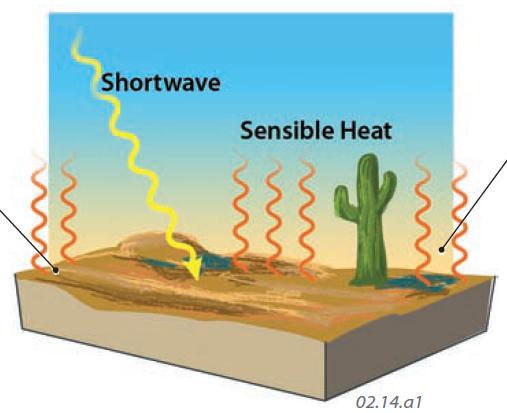
Shortwave Radiation Converted to Sensible Heat
1. Some energy that strikes Earth's surface is absorbed by molecules, increasing their temperature, an increase in sensible heat. The heat stored in the land and water is called ground heat. The amount of heating of the surface, and the flux of ground heat, depends on all the factors that influence the distribution of insolation, such as latitude, season, length of day, and cloud cover. It is also influenced by whether insolation strikes land or water, by moisture and mineral content of the surface, and other factors.
2. As Earth's land and water transfer sensible heat to the adjacent atmosphere, the air warms. Heating of Earth's land and water therefore warms the adjacent atmosphere — actually more than direct insolation does. The land and water surfaces heat up and cool down slowly. It takes both a while to exchange heat with the air, so warmest surface temperatures of the day typically occur hours after noon. For the same reason, coldest temperatures typically occur just before sunrise.
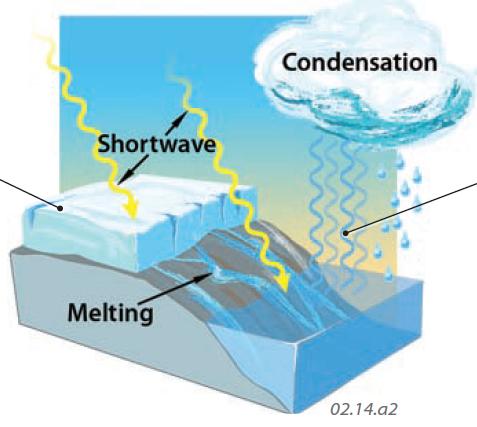
Shortwave Radiation Converted to Latent Heat
3. Energy striking Earth's surface can also be converted to latent heat as ice melts. As the shortwave energy of the insolation converts ice to liquid, molecules in the liquid begin to carry the associated latent heat of fusion. The water can flow away from the site of melting, or can remain and refreeze, releasing the latent heat to the local environment.
4. Shortwave energy from insolation can also cause water to evaporate, forming water vapor. Molecules in the vapor carry the latent heat of vaporization into the atmosphere. When the vapor condenses into water drops, such as in clouds or as precipitation, the molecules give back this latent heat to the surrounding atmosphere, warming it (sensible heat). Through this process, shortwave radiation is converted first to latent heat and then to sensible heat. Similar processes include conversion of ice to vapor (sublimation) and vapor to ice (deposition).
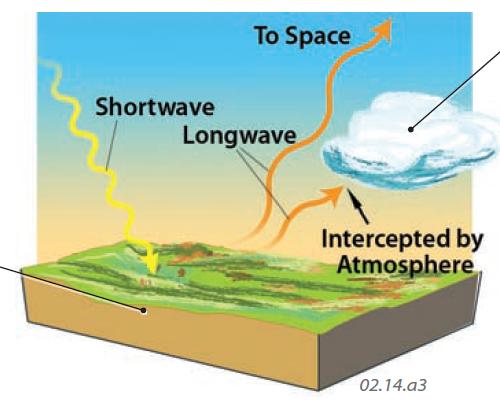
Shortwave Radiation Converted to Longwave Radiation
5. Shortwave radiation that strikes the surface can be absorbed by materials it encounters, such as rocks, soils, wood, and water, increasing the motions of their constituent molecules. These materials emit some of this energy and are at temperatures appropriate for the energy emissions to be at long wavelengths (according to Wien's Law). In this way, shortwave radiation from the Sun is absorbed by the surface and then radiated as longwave radiation.
6. The emitted longwave radiation can return to space or be intercepted by clouds, gas molecules, or solid aerosols. Some of the energy absorbed by these atmospheric components is then re-emitted as longwave radiation in all directions — into space, into other parts of the atmosphere, or back toward Earth's surface. Longwave energy that is emitted downward is commonly called counter-radiation, and helps keep the Earth at temperatures suitable for life.
How Do Greenhouse Gases Interact with Electromagnetic Energy?
The atmosphere contains certain components, most notably water molecules, that absorb some wavelengths of outgoing longwave radiation (OLR). This energy is then emitted via longwave radiation to the surrounding atmosphere or even back down to Earth, keeping the planet warmer than it would be otherwise. Many people use the term greenhouse effect to refer to this warming influence, such as the way glass allows in sunlight but traps heat to keep a greenhouse warm. Atmospheric components that exhibit this behavior are called greenhouse gases.
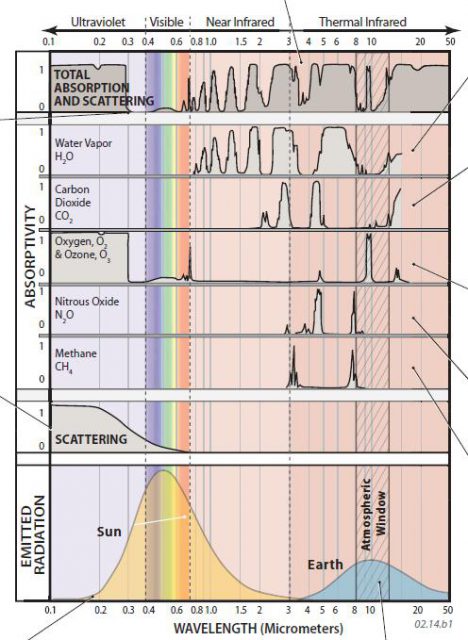
- The figure below shows absorption (vertical axis of graph) of EMR at various wavelengths (horizontal axis), by individual greenhouse gases. It also shows the amount of radiation that is scattered by gases and particles (second graph from bottom). Peaks on each curve show where the gas absorbs a certain wavelength of energy. A value of 1 indicates very high absorption or scattering at that wavelength. The top graph shows the composite effect for all gases in the atmosphere.
- Characteristic wavelengths of incoming visible light and adjacent wavelengths of UV are barely impacted by atmospheric gases. As a result, insolation at these wavelengths is mostly transmitted through the atmosphere, illuminating and heating Earth's surface.
- Insolation at shorter wavelengths, such as UV, is nearly all absorbed by atmospheric gases, especially by oxygen gas, ozone, and scattering by particles. These atmospheric gases and particles shield us from this dangerous radiation.
- The two shaded curves on the bottom of the diagram show the wavelengths of energy emitted by the Sun (yellow) versus the Earth (blue). The majority of insolation entering the top of Earth's atmosphere is in the form of visible light (0.4 – 0.7 mm), whereas Earth emits its radiation back to space in the form of longwave radiation, mostly as thermal infrared, at wavelengths between about 8 and 20 mm. There is a clear separation between the wavelengths of incoming shortwave radiation and those of outgoing longwave radiation. The curves for individual gases show that each greenhouse gas intercepts some amounts of certain wavelengths of outgoing longwave radiation.
- There is a band of frequencies at 8 – 13 mm that pass through the atmosphere without much loss from scattering and other effects. This is expressed as the large trough in the top curve, which shows the total effect of absorption and scattering for the entire atmosphere. The trough represents a part of the thermal IR spectrum where most energy is transmitted through the atmosphere — this region of the longwave spectrum is called the atmospheric window because it allows most longwave radiation out through the atmosphere.
- Water vapor (H2O) is an abundant, and the most important, greenhouse gas. It absorbs a wide range of wavelengths of OLR, including most wavelengths greater than 15 mm. The quantity of atmospheric water vapor varies from place to place and from time to time. It is typically higher over the oceans than over land. Water vapor and clouds help the surface stay warm by emitting extra counter-radiation downward, moderating changes in temperature between day and night.
- Carbon dioxide (CO2) is an atmospheric gas present in trace amounts, measured in parts per million. Quantities of CO2 in the atmosphere have increased as the world warmed from the last glacial advance. Some CO2 released into the atmosphere is from natural sources, such as volcanic eruptions and the natural decay of vegetation.
- Oxygen and ozone intercept only some wavelengths of OLR. The previously noted ability of these gases to effectively absorb incoming UV radiation can be seen clearly by high peaks on the far left side of this diagram.
- Nitrous oxide (N2O) is a by-product of many industrial chemical processes, particularly production of fertilizers. It absorbs some energy at thermal-IR wavelengths.
- Methane (CH4), sometimes known as “marsh gas,” occurs naturally as the result of the decay of organic material. Methane can also be released from oil and gas production or when frozen ground thaws or the seafloor warms up. It absorbs some thermal-IR energy.