What Is Latent Heat?
WATER OCCURS IN ALL THREE PHYSICAL STATES —solid, liquid, and gas — at temperatures commonly found on Earth. Although the chemical structure of water remains unchanged from state to state, the three states, also called phases, are differentiated by the physical spacing of the water molecules. Considerable quantities of energy, contained as latent heat, are involved in these changes of state, and act as moderators of global climate.
What Are the Forms of Latent Heat?
The chemical substance water consists of two hydrogen atoms bonded to a single oxygen atom, with a chemical formula of H2O. The change in state between any two of these phases requires the addition of energy or involves the release of energy, depending in which direction the change is occurring (e.g., liquid to solid versus solid to liquid).
1. When ice is placed in warmer surroundings, like an ice cube on a kitchen counter, energy from its environment flows into the ice, increasing the internal motions of water molecules in the solid, crystalline structure. At first the ice heats up (increases in temperature), but once it reaches a certain temperature (its melting point) it begins to melt. Melting requires energy to be added (as shown by the blue-to-red color of the arrow for melting).
2. During melting, energy input into the system is stored (absorbed) in water molecules of the liquid — as latent heat. The latent heat associated with melting is called the latent heat of fusion. If enough energy is removed for the liquid water to be converted back into ice during freezing, the latent heat stored in the water molecules is released back into the surroundings as heat (also called latent heat of fusion). For freezing to continue, this released energy must be dispersed into the surroundings. The warm flow of air coming from the back or bottom of a household freezer is the heat being dispersed from the cooling system.
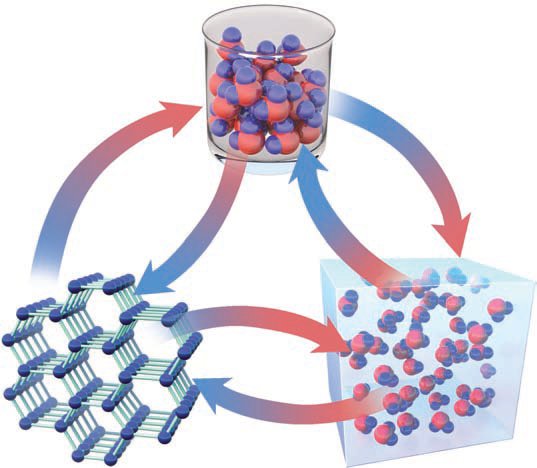
3. Conversion from a liquid phase to a vapor phase, evaporation, also requires an input of energy from the surroundings, as when you boil water in a pan. During evaporation, energy added to the liquid breaks the bonds holding the water molecules together, allowing molecules to escape as a gas. The liberated gas molecules are moving fast and so have increased kinetic energy and carry energy stored as latent heat. When the gas molecules are cooled and recombine into a liquid , the process of condensation, the latent heat is released back into the surroundings. The latent heat associated with evaporation and condensation is the latent heat of vaporization.
4. Water can also go directly from the solid state to a vapor, the process of sublimation. Sublimation requires energy from the surroundings and stores latent heat in the gas molecules — the latent heat of sublimation. The reverse process, converting water vapor directly into ice, is called deposition and is the main way snowflakes form. Deposition releases the latent heat back into the environment, but when it is snowing it is cold enough that we would not easily notice any addition of latent heat to the cold air.
What Happens to Temperature During Melting and Boiling?
1. An interesting thing happens to temperature when we heat ice and convert it to liquid water and then water vapor, as shown by the graph to the right. This graph plots the energy input into the system (measured in a unit of energy called a kilojoule) versus the resulting temperature if we start with a kilogram of ice. The process begins in the lower left corner, with ice at ?20°C, well below its melting temperature (0°C).
2. The initial input of energy into the system causes ice to increase in temperature, represented by the first inclined, red part of the line. The increase in energy is expressed as sensible heat.
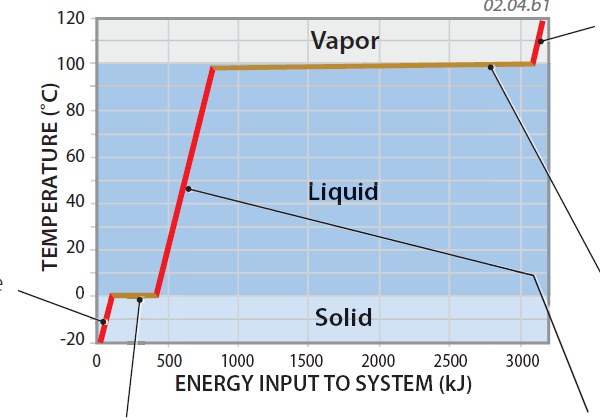
3. When the temperature reaches 0°C, the melting point, the ice starts to melt. The temperature does not change during melting (as shown in the short, horizontal brown line). Instead, all the increase in energy is going into breaking the bonds and is stored as latent heat.
4. Once all the ice is melted, the increase in energy again causes an increase in temperature (sensible heat), as shown by the second inclined red line. The temperature of the water increases until it reaches the boiling point (100°C).
5. As the water starts to boil, it does not increase in temperature (long, horizontal brown line). All increase in energy is used to convert liquid into the vapor phase and is stored as latent heat.
6. Once all the water has become vapor, further heating causes the steam to increase temperature (inclined, red line). In this entire process, more energy was used to change states (brown horizontal lines) than was used to increase temperature (the inclined, red lines). Of the total amount of energy used, less than 20% went to change temperature and more than 80% was used to change state!
What Does Latent Heat Do to the Surroundings?
Latent energy added to the water molecules or released by the water molecules allows the phase change to proceed, but it also impacts the temperature of the environment. More than five times the energy is involved in evaporating or condensing water (the latent heat of vaporization) than in raising the temperature of the same mass of water from the freezing point to the boiling point. The large quantities of latent heat have a huge role in many aspects of our world, including changes in atmospheric temperature.
Water: Vapor, Liquid, and Ice
This graph shows conditions under which each phase occurs. For reference, a pressure of 1.0 bar is the average atmospheric pressure at sea level, and 25°C is standard room temperature.
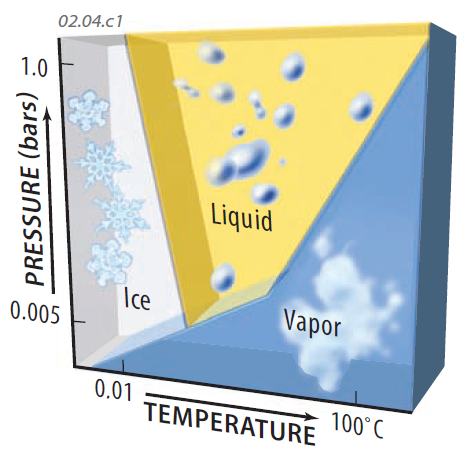
Ice occurs at low temperatures, whereas liquid and water vapor are favored by higher temperatures. Higher pressure acts to hold the water molecules within the liquid rather than allowing them to escape into the air. If we cool vapor, we get liquid at higher pressures or ice or at lower ones, causing the formation of clouds or precipitation, both of which consist of liquid (drops of water) and ice.
Phases, such as vapor, that require more energy to form are called high-energy states, whereas less energetic ones are low-energy states.
Cooling and Heating the Air
When water in the atmosphere changes state, it releases or takes in thermal energy, heating or cooling the surrounding air. This diagram illustrates the change in air temperature during phase changes. Red arrows indicate that phase change in this direction causes the surrounding air to heat up. Blue arrows indicate that the surrounding air must provide heat to the phase change, and so the air cools.
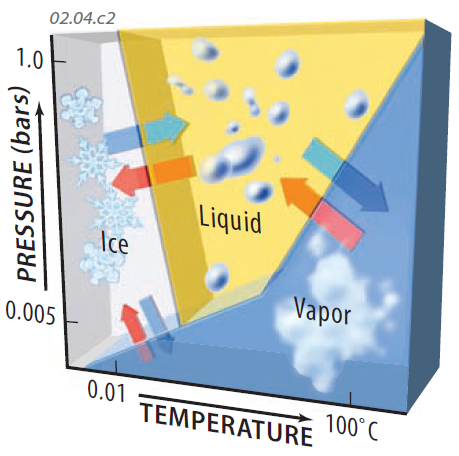
Energy must be released into the surroundings for water to go from a higher-energy state to a lower-energy molecular state. Heat is released (red arrows) when water vapor forms droplets or ice crystals, or when liquid water freezes — this warms the surrounding air. Heat is taken in from the surroundings (blue arrows) when ice melts or water evaporates, or ice sublimates. Any of these processes cool the air.
What Are Examples of Latent Heat in the Environment?
1. In 2004 Hurricane Ivan deposited an average of 15 cm of rainfall over Duval County and the city of Jacksonville in northeastern Florida. When we consider the amount of rainfall, the area of the county, and the typical amount of latent heat released during condensation, we calculate that this storm, in this one county, released latent heat equivalent to much more than all the electrical energy consumed by the world since electricity was harnessed for human use. Many such storms occur each year and cover even larger areas.

2. Severe freezes are a serious threat to the citrus industry in the southern and southwestern U.S. Sustained subfreezing temperatures may ruin the crop and even destroy the tree. To prevent extensive losses, grove owners spray the trees with water, which freezes on the crop. At first sight the weight of the accumulated ice only seems to add to the damage by breaking off fruit and limbs, but each kilogram of water that freezes on the trees releases enough energy to the fruit and plant to prevent cold temperatures from destructively freezing water within the cells of the plant.

3. A cold beverage in a can, bottle, or glass warms as a result of conduction from the cold container and its warmer surroundings, like a table, but it warms even more quickly because condensation releases latent heat on the outside of the container.
