Humidity
Blistering summer heat waves can be deadly, with the elderly and the ill at most risk. However, even healthy young people need to be careful, especially in hot, humid weather. High humidity slows the evaporation of perspiration from our bodies, reducing its cooling effect. Clearly, it is not only the temperature of the air that controls how hot weather affects us—the amount of water vapor in the air is important as well.
The amount of water vapor present in air, referred to as humidity, varies widely from place to place and time to time. In the cold, dry air of arctic regions in winter, the humidity is almost zero, while it can reach up to as much as 3 to 4 percent of a given volume of air in the warm wet regions near the Equator.
An important principle concerning humidity states that the maximum quantity of water vapor an air parcel can contain is dependent on the air temperature itself. Warm air can contain more water vapor than cold air—a lot more. Air at room temperature (20°C, 68°F) can contain about three times as much water vapor as freezing air (0°C, 32°F).
SPECIFIC HUMIDITY
The actual quantity of water vapor contained within a parcel of air is known as its specific humidity and is expressed as grams of water vapor per kilogram of air (g/kg). The equation for specific humidity is given as:
specific humidity = mass of water vapor / mass of total air
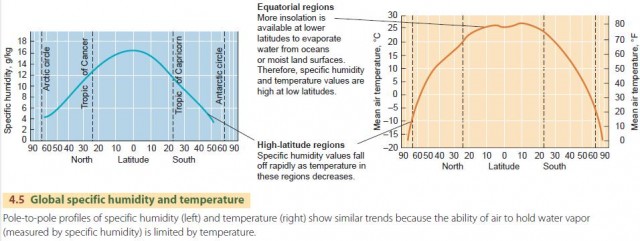
Specific humidity is often used to describe the amount of water vapor in a large mass of air. Both humidity and temperature are measured at the same locations in standard thermometer shelters the world over and also on ships at sea. Specific humidity is largest at the warm, equatorial zones, and falls off rapidly toward the colder poles (Figure 4.5). Extremely cold, dry air over arctic regions in winter may have a specific humidity as low as 0.2 g/kg, while the extremely warm, moist air of equatorial regions often contains as much as 18 g/kg. The total natural range on a worldwide basis is very wide. In fact, the largest values of specific humidity observed are from 100 to 200 times as great as the smallest values.
DEW-POINT TEMPERATURE
Although the actual amount of water in a given volume of air is called the specific humidity, this is not the same as the maximum quantity of moisture that a given volume of air can contain at any time. This maximum specific humidity, referred to as the saturation-specific humidity, is dependent on the air's temperature.
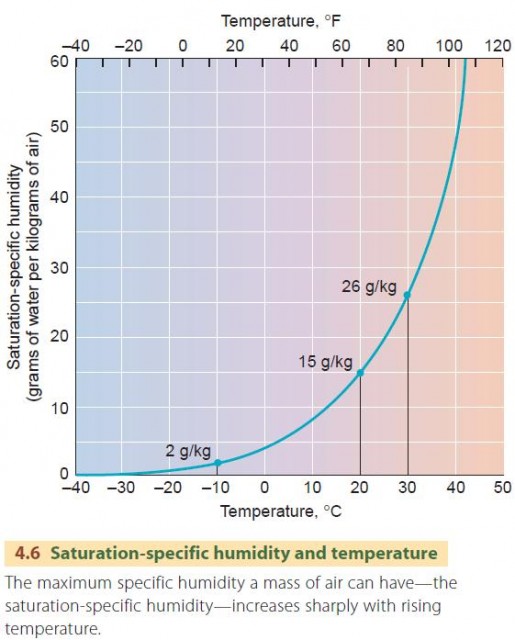
In Figure 4.6 we see, for example, that at 20°C (68°F), the maximum amount of water vapor that the air can contain—the saturation-specific humidity—is about 15 g/kg. At 30°C (86°F), it is nearly doubled— about 26 g/kg. For cold air, the values are quite small. At –10°C (14°F), the maximum is only about 2 g/kg. Another way of describing the water vapor content of air is by its dew-point temperature, also called simply the dew point. If air is slowly chilled, its saturationspecific humidity decreases. This can continue until the saturation-specific humidity is equal to the specific humidity. When this condition is reached, the air has reached saturation, because the air contains the maximum amount of water vapor possible. If further cooling continues, condensation begins. The temperature at which saturation occurs is therefore known as the dewpoint temperature—that is, the temperature at which dew forms by condensation.
RELATIVE HUMIDITY
When weather forecasters speak of humidity, they are usually referring to relative humidity. This measure compares the amount of water vapor present to the maximum amount that the air can contain at its given temperature. The relative humidity is expressed as a percentage given by:
relative humidity = 100 ? specific humidity / saturation-specific humidity
For example, if the air currently contains half the moisture possible at the present temperature, then the relative humidity is 50 percent. When the humidity is 100 percent, the air contains the maximum amount of moisture possible. The air is saturated, and its temperature is at the dew point. When the specific humidity and saturation-specific humidity are not the same, the air is unsaturated. Generally, when the difference between the two is large, the relative humidity is small and vice versa.
The relative humidity of the atmosphere can change in one of two ways. First, the atmosphere can directly gain or lose water vapor, thereby changing the specific humidity of the air mass. For example, additional water vapor can enter the air from an exposed water surface or from wet soil. This process is slow because the water vapor molecules must diffuse upward from the surface into the air layer above.
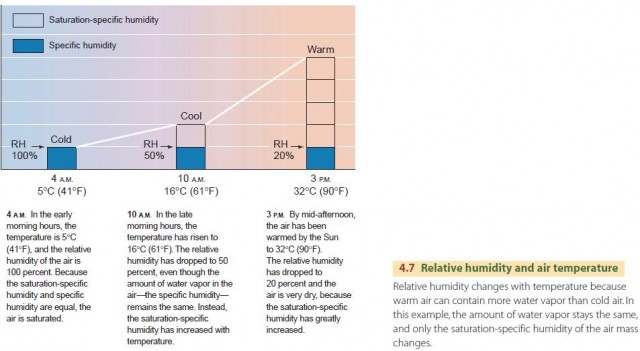
The second way relative humidity changes is through a change of temperature. Even though no water vapor is added, an increase of temperature results in a decrease of relative humidity (Figure 4.7). Recall that the saturation-specific humidity of air is dependent on temperature. When the air is warmed, the saturation-specific humidity increases. The existing amount of water vapor, given by the specific humidity, then represents a smaller fraction of the saturation-specific humidity.
A simple method of measuring relative humidity uses two thermometers mounted together side by side in an instrument called a sling psychrometer. After whirling the psychrometer in the air, the temperature difference between the wet-bulb thermometer and the dry-bulb thermometer can be used to derive the relative humidity. Direct-reading electronic instruments are also available for measuring humidity.

- Water in the Environment
- Acid Deposition
- Global Warming and the Greenhouse Effect
- World Patterns of Air Temperature
- Daily and Annual Cycles of Air Temperature
- Temperature Structure of the Atmosphere
- Surface and Air Temperature
- Carbon Dioxide — On the Increase
- CERES—Clouds and the Earth’s Radiant Energy System