Why Do Temperatures Vary Between Oceans and Continents?
WATER EXHIBITS VERY DIFFERENT thermal properties from those displayed by the rocks and soil of land. These differences in thermal properties cause oceans and land to warm and cool at different rates, leading to significant temperature variations between oceans and land. Such differences help explain major patterns of global temperature and climate.
How Do Water and Earth Materials Respond to the Same Changes in Energy?
Heat Capacity
1. In evaluating how water, rocks, and other materials heat up or cool down, an important consideration is how much energy is needed to heat up an object, and how much heat that object can retain. A physical attribute called heat capacity expresses how much heat is required to change a volume's temperature by one Kelvin.

2. The heat capacity of an object is determined by the kind of material in the object, such as rock versus water, and by the size of the object. The larger block above has a higher heat capacity than the smaller block, as long as both are composed of similar materials.
Specific Heat
3. To compare the inherent thermal responses of different materials, irrespective of how much of the material is present, we use a property called specific heat capacity, or simply specific heat. Specific heat is the amount of energy needed to increase a kilogram mass of a substance by 1 K (or 1 C°).

4. The specific heat of water is four times that of most rocks and materials. This means that it takes four times more energy to heat water than it takes to heat an equivalent mass of rock.
Thermal Responses of Water Versus Other Earth Materials
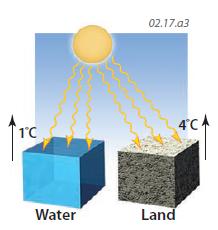
5. Due to their differences in specific heat, oceans (water) heat up during the day differently than land. If the same amount of insolation strikes water and land, the land will increase in temperature 4 degrees (K or C°) for every degree the water increases. As a result, land warms up much faster than the ocean, under the same environmental conditions, but some of this difference is offset by increased losses of heat from the land to the air.
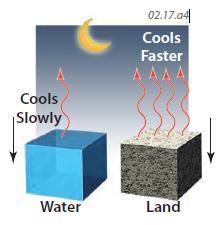
6. At night, the water and land both lose energy to the cool night air. For the same energy loss, land cools more than does water. Also, the land became hotter during the day, and hot objects radiate more energy than cool ones, so the land loses energy faster than the water. As a result, land cools off much faster than water at night.
Depth of Heating, Cooling, and Mixing
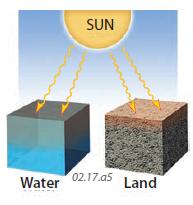
7. Another factor in how water and land respond to changes in insolation and air temperatures is how deeply heat is able to enter each material. Nearly all water allows at least some transmission, so shortwave radiation can penetrate to depths of tens of meters or more. In contrast, rocks and soil are largely opaque, so insolation is confined to the surface, and heat must move downward into the land by conduction, which it does by only a meter or two during the day.
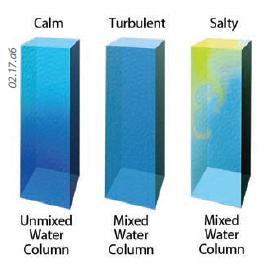
8. Some materials, such as water, are relatively mobile, which allows them to flow and mix. Under calm ocean conditions (left column), limited mixing causes surface waters warmed by the Sun to remain near the surface, so there is a strong temperature contrast with depth. Surface winds induce waves (center column), resulting in turbulence, which carries warm waters downward, mixing them with cooler waters at a depth. Salt water (right column) is more dense than fresh water, so any waters that are saltier than normal, such as from partial evaporation, can sink, causing mixing of the water column. Mixing allows heat to be carried deeper into the water column (much faster than heat is conducted) and brings up cooler water that gives off energy to the atmosphere more slowly (because it is cool). As a result, mixed water heats up more slowly than does land, which experiences almost no vertical mixing.
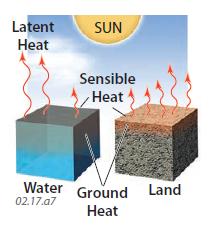
Latent Heat
9. Water has another unique capacity relative to land — it can store abundant energy as latent heat. Insolation that strikes water is transformed into one of three different fluxes of energy. Some energy goes into heating the water (ground heat), some heats the air (sensible heat in the atmosphere), but a large amount goes into latent heat produced by evaporation. In contrast, insolation striking land goes almost entirely into sensible heat in the atmosphere and into ground heat. Land contains some water, but lesser amounts of its insolation goes into latent heat.
Distribution of Continents and Oceans
10. Another factor that influences the global energy budget, and the balance of energy that falls on land versus the oceans, is the difference between the Northern and Southern Hemispheres. As can be observed on any map or globe, the Northern Hemisphere has the majority of the planet's landmasses, whereas the Southern Hemisphere is dominated by oceans. As a result, an equal amount of insolation striking both hemispheres will result in more latent heat being generated in the Southern Hemisphere than in the Northern Hemisphere. In December, when the Southern Hemisphere more directly faces the Sun, more insolation will fall on water than during June.
How Do Temperatures Reveal Thermal Differences Between Ocean and Land?
1. These various factors, from specific heat to latent heat, cause land and water to respond very differently to insolation and to the change from day to night. Land, with its relatively low specific heat, limited mixing, and limited amount of latent heating, warms up more quickly than water and reaches higher temperatures. At night, land's higher daytime temperatures cause it to lose heat more rapidly than does water. This keeps the night warm for a while, but eventually the cool night air dominates.
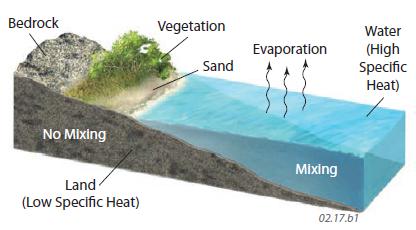
2. Water, with its high specific heat, partial transparency, and ability to mix vertically, heats up more slowly and does not reach as high a temperature. Also, much insolation is converted into latent heat that is transferred to the atmosphere via evaporation and condensation, so this energy is not available to heat the body of water. Large bodies of water therefore experience smaller temperature variations and more moderate temperatures overall, compared to land. Land areas adjacent to the water can partly experience the moderate temperatures caused by the unique thermal properties of water.
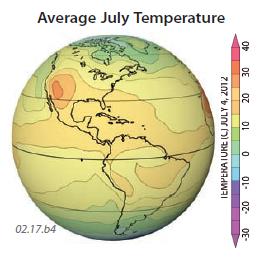
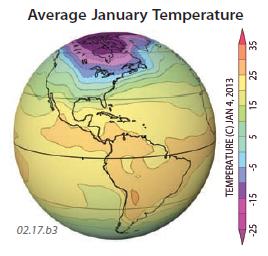
3. These three globes show the yearly average temperature, average January temperature, and average July temperature, all for 2011. Observe the temperatures shown on each globe and use concepts presented in this chapter to try to explain the main patterns. Aspects to consider include variations in insolation due to latitude and clouds, land-sea contrasts, and the distribution of continents. These globes show data for the different times of year, so they express seasonal variations. In each globe, red and orange are hotter, blue and purple are colder, and yellow and green are intermediate in temperature. The overall average temperatures range from less than ?50°C in Antarctica to locally greater than +20°C in some tropical and subtropical regions.
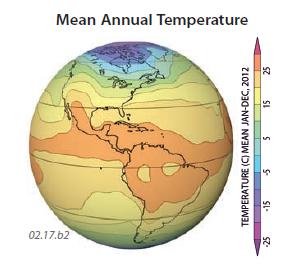
4. Average January temperatures go from less than ?50°C near the North Pole to more than +25°C in the tropics.
5. Average July temperatures are higher in the Northern Hemisphere than in the Southern Hemisphere.