The Adiabatic Process
What makes the water vapor in the air turn into liquid or solid particles that can fall to the Earth? The answer is that the air is naturally cooled. When air cools to the dew point, the air is saturated with water. Think about extracting water from a moist sponge. To release the water, you have to squeeze the sponge—that is, reduce its ability to hold water. In the atmosphere, chilling the air beyond the dew point is like squeezing the sponge—it reduces the amount of water vapor the air can contain, forcing some water vapor molecules to change state to form water droplets or ice crystals.
One mechanism for chilling air is nighttime cooling. On a clear night, the ground surface can become quite cold as it loses longwave radiation. If the air is moist, frost can be deposited as water vapor forms ice crystals. However, this cooling is not enough to form precipitation. Precipitation only forms when a substantial mass of air experiences a steady drop in temperature below the dew point. This happens when an air parcel is lifted to higher and higher levels in the atmosphere.
DRY ADIABATIC RATE
If you have ever pumped up a bicycle tire using a hand pump, you might have noticed that the pump gets hot. If so, you have observed the adiabatic principle. This important law states that if no energy is added to a gas, its temperature will increase as it is compressed. As you pump vigorously, compressing the air, the metal bicycle pump gets warm. Conversely, when a gas expands, its temperature drops by the same principle. Physicists use the term adiabatic process to refer to a heating or cooling process that occurs solely as a result of pressure change, with no heat flowing into or away from a volume of air.
How does the adiabatic principle relate to the uplift of air and to precipitation? The missing link is simply that atmospheric pressure decreases as altitude increases. As a parcel of air is uplifted, atmospheric pressure on the parcel becomes lower, and the air expands and cools, as shown in Figure 4.9. As a parcel of air descends, atmospheric pressure becomes higher, and the air is compressed and warmed.
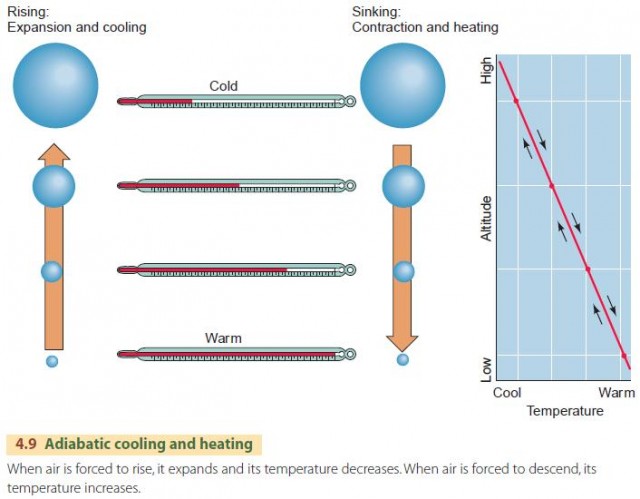
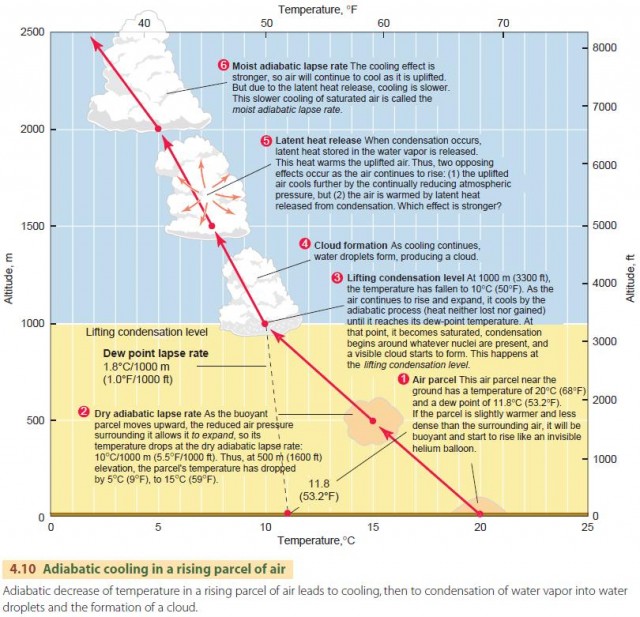
We describe this behavior in the atmosphere using the dry adiabatic lapse rate, as shown in the lower portion of Figure 4.10. It applies to a rising air parcel that has not yet been cooled to saturation. The dry adiabatic lapse rate has a value of about 10°C per 1000 m (5.5°F per 1000 ft) of vertical rise. That is, if a parcel of air is raised 1 km, its temperature will drop by 10°C. Conversely, an air parcel that descends will warm by 10°C per 1000 m. This is the dry rate because no condensation occurs during this process.
There is an important difference to note between the dry adiabatic lapse rate and the environmental temperature lapse rate. The environmental lapse rate is simply an expression of how the temperature of still air varies with altitude. This rate will vary from time to time and from place to place, depending on the state of the atmosphere. It is quite different from the dry adiabatic lapse rate. The dry adiabatic lapse rate applies to a mass of air moving vertically. It does not vary with time and place, and it is determined by physical laws, not the local atmospheric state.
MOIST ADIABATIC RATE
Let's continue examining the fate of a parcel of air that is moving upward in the atmosphere (Figure 4.10). As the parcel moves upward, its temperature drops at the dry adiabatic rate, 10°C/1000 m (5.5°F/1000 ft). Note, however, that the dew-point temperature changes slightly with elevation. Instead of remaining constant, it falls at the dew-point lapse rate of 1.8°C/1000 m (1.0°F/1000 ft).
As the rising process continues, the air is eventually cooled to its dew-point temperature, and condensation starts to occur. This is shown in Figure 4.10 as the lifting condensation level. The lifting condensation level is thus determined by the initial temperature of the air and its initial dew point
and can differ from the example shown here. If the parcel of saturated air continues to rise, a new principle comes into effect—latent heat release.
That is, when condensation occurs, latent heat is released by the condensing water molecules and warms the surrounding air molecules.
In other words, two effects are occurring at once. First, the uplifted air is being cooled by the reduction in atmospheric pressure. Second, it is being warmed by the release of latent heat from condensation.
Which effect is stronger? As it turns out, the cooling effect is stronger, so the air will continue to cool as it is uplifted. However, because of the release of latent heat, the cooling will occur at a lesser rate. This cooling rate for saturated air is called the moist adiabatic lapse rate and ranges between 4 and 9°C per 1000 m (2.2–4.9°F per 1000 ft). Unlike the dry adiabatic lapse rate, which remains constant, the moist adiabatic lapse rate is variable because it depends on the temperature and pressure of the air and its moisture content. For most situations, however, we can use a value of 5°C/1000 m (2.7°F/1000 ft). In Figure 4.10, the moist adiabatic rate is shown as a slightly curving line to indicate that its value changes with altitude.
Keep in mind that as the air parcel becomes saturated and continues to rise, condensation is occurring. This condensation produces liquid droplets and solid ice particles that form clouds and eventually precipitation.