How Does Precipitation Form?
THE PROCESS OF PRECIPITATION is vital to life on Earth, helping to redistribute water from the oceans to the atmosphere to the land. Precipitation is the ultimate source of all the fresh water on the planet, which we depend on in our daily lives. How does precipitation occur? What is going on inside clouds that forms raindrops, snowflakes, and hail? It turns out there are two important mechanisms by which precipitation droplets form, one of which is somewhat surprising.
What Is Precipitation?
1. Precipitation is the process whereby liquid droplets of water (raindrops), solid bits of ice (snowflakes and hail), or some combination of these fall from the sky. Examine this figure and consider all the processes that have to occur to cause rain, snow, or hail.
2. The cycle begins with evaporation of water in the oceans and other parts of Earth's surface, a process that puts water vapor into the atmosphere. Next, the water vapor forms the various types of clouds, which can contain tiny drops of water, ice crystals, or some of each.
3. For precipitation, some processes are occurring within the cloud that make the water droplets or bits of ice heavy enough that the pull of gravity can overwhelm the buoyancy forces (atmospheric instability) that uplift air within the cloud (the rising air is how most clouds form).
4. Whether a cloud contains drops of liquid, ice crystals, or some combination depends primarily on the temperature of the cloud. If ambient temperatures near the cloud are warm, the cloud will contain mostly drops of liquid. Temperatures decrease upward within a cloud, however, so the upper levels can contain ice, while the lower levels contain drops.
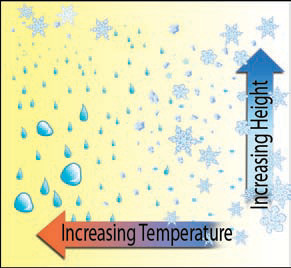
5. Under cold ambient conditions, a cloud contains mostly ice throughout its vertical extent. Clouds at intermediate ambient temperature, or intermediate altitudes, will contain a mix of drops and ice.
How Do Water Droplets Form and Grow?
1. An important factor in how water droplets in a cloud grow to become raindrops is the immense differences in size between the various players. Raindrops are huge compared to the size of water droplets that form a cloud, as shown by their relative sizes in the properly scaled diagram below.
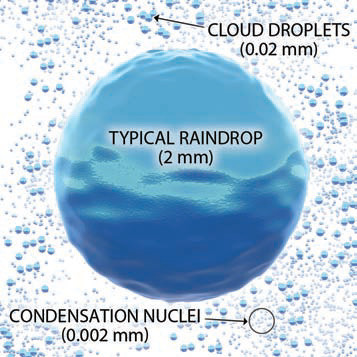
2. It is energetically difficult for the tiny water droplets in clouds to just form by themselves, but it is easier for them to form if they condense around even tinier particles, such as dust, salt, and smoke. Due to this role, such particles are called condensation nuclei.
3. The figure to the right illustrates what can happen to a moving water drop that interacts with smaller cloud droplets around it.
4. Some larger drops form when liquid water droplets in clouds merge and grow to a size that can be pulled down by gravity. A water droplet begins to fall as soon as the downward-directed gravitational force exceeds the upward-directed buoyancy force caused by instability. This occurs sooner for larger droplets than for smaller droplets, and the larger drop overtakes the smaller droplets on their descent, making the falling drop even larger. Eventually it falls as a raindrop. The growth of a drop in this manner is called collision-coalescence.
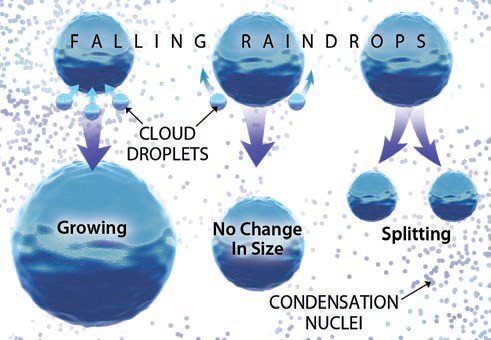
5. In other cases, the smaller droplets simply slide past the falling drop, because the collision is not enough to break the surface tension that tends to keep each drop intact. In this case, the falling drop will not increase in size as it falls. This situation is actually very likely under many atmospheric conditions.
6. Alternatively, wind resistance can reshape the falling drop until it becomes easier to break the drop into separate drops than to retain its contorted shape. In this way, a falling raindrop becomes smaller, which can also occur if water molecules on the outside of the drop simply evaporate into the surrounding air.
How Do Ice, Liquid, and Vapor Interact Within a Cloud?
Many clouds are in environments in which the temperature is below freezing, even in summer. Exceptions are clouds in the tropics and low in the atmosphere, or a cloud being warmed by latent heat during precipitation. Even when temperatures are below freezing, some liquid water droplets exist. This sets up a situation in which water exists in all three phases — vapor, liquid, and ice (solid). The interaction between these three coexisting phases allows precipitation to form much more easily than would otherwise be possible if only vapor and liquid were present
1. The diagram to the right shows the familiar saturation curve for water vapor, but focuses on conditions slightly above and slightly below freezing. At temperatures below freezing, the saturation curve for water vapor near ice (the dashed line) diverges from that for water vapor and liquid (the solid line). The divergence of the two curves begins at the freezing point because ice can't easily exist above freezing.
2. The area between the two curves, shaded orange, depicts temperatures at which air is unsaturated with water vapor relative to a liquid but above saturation for air next to ice in the cloud. If a parcel of air has the water-vapor capacity and temperature to plot in this orange area of the plot, vapor will become deposited on ice crystals while liquid water drops are evaporating at the same time.
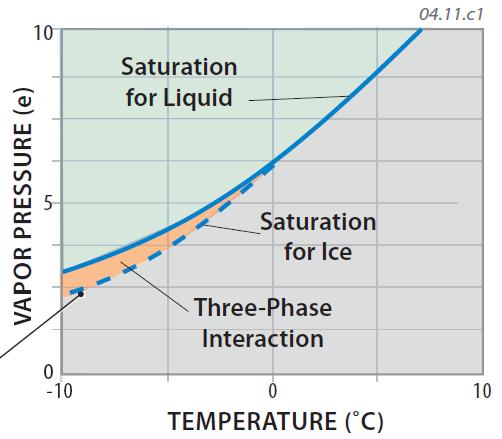
3. Another way to state this is that saturation is reached with slightly less water vapor when air is in contact with ice in the cloud than when it is in contact with liquid water, even at the same temperature. That is, less water vapor can exist in air that is in contact with ice than in air that is in contact with liquid water, if both are at the same temperature.
4. This difference in saturation levels aids in the formation of precipitation. If ice, water vapor, and drops of liquid water coexist at the conditions specified by the orange field in the previous figure, then the air will be unsaturated next to the liquid drop. As a result, water molecules will evaporate from the drops, increasing the relative humidity of the surrounding air.
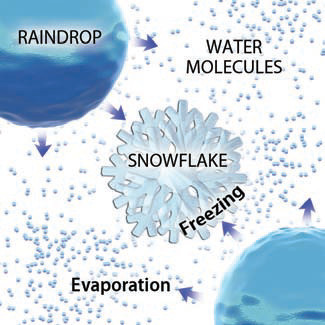
5. As the liberated water vapor molecules diffuse into the air, they cause an increase in water vapor adjacent to a nearby snowflake. The air near the snowflake therefore becomes saturated or supersaturated with respect to the ice. This in turn causes water vapor molecules to be deposited on the snowflake, enlarging it.
6. Depending on the air temperature, the ice can remain as a solid or if temperature increases slightly, the ice can melt, going directly from ice to liquid. In this way, liquid water becomes water vapor, which then becomes ice, which then becomes drops, an easier path (under many conditions) than trying to overcome surface tension to grow drops or the difficult energetics of nucleation required to make new drops. This process of precipitation is often called the Bergeron process after one of the scientists who discovered it.
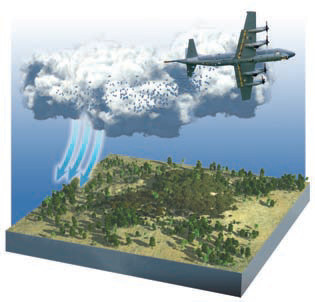
How Cloud Seeding Works
Technology exists to “help” precipitation occur more efficiently in droughtstricken areas. Strategies generally involve injecting particles into clouds to enhance droplet or ice crystal growth, either via a ground-based delivery system or more commonly airplanes that fly through the clouds, as shown here. One strategy uses dry ice to cool the cloud to temperatures so low that vapor deposits onto ice more readily. A second strategy is to inject into the cloud microscopic solid particles that act as condensation nuclei. The idea is that water vapor can condense or deposit much more easily when it has something to “hold onto” during the phase change. Silver iodide has often been used for this purpose because its crystalline structure maximizes opportunities for vapor to attach to it. These cloud seeding efforts have achieved mixed results. In some cases, precipitation has been enhanced or shifted to other areas. Interventions into natural processes, however, often have unintended consequences, such as, in this case, silver-iodide pollution from the chemicals used to seed the cloud.