What Is Air Pressure?
PRESSURE OF GASES WITHIN THE ATMOSPHERE is highly variable, both vertically and laterally. These variations in pressure define the structure of the atmosphere and also determine the nature and direction of atmospheric motions. If one place in the atmosphere has higher pressure than another place, this imbalance of pressure (and therefore also atmospheric mass) must be evened out, causing the air to flow. How do we describe and measure pressure, and how do we use these measurements to understand or even predict the flow of air?
What Is Pressure?
Pressure is an expression of the force exerted on an area, usually from all directions. In the case of a gas, pressure is related to the frequency of molecular collisions, as freely moving gas molecules collide with other objects, such as the walls of a container holding the gas. It is such collisions that keep a balloon, soccer ball, or bicycle tire inflated.
1. Molecules of gas in a sealed glass container move rapidly in random directions, and some strike the walls of the container. The force imparted by these collisions is pressure. The more collisions there are, the more pressure is exerted on the walls of the container.
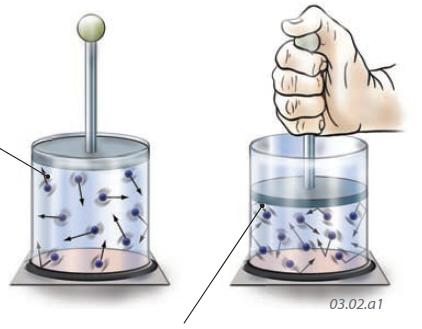
2. If we push down on the lid of the container, the same number of molecules are confined into a smaller space. Lower parts of the container walls are now struck by a greater number of the more closely packed gas molecules, so the pressure is greater. Decreasing the volume of a gas increases its pressure, consistent with Boyle’s Law.
3. What happens if we put a weight on top of the lid (center container) and then either cool or heat the gas in the container?
4. If we cool the container by placing it in ice, the molecules become less energetic and so strike the walls and lid of the container less often — the gas pressure decreases and the lid moves down.

5. If we instead heat the container, the gas molecules become more energetic and strike the walls and lid of the container more often — the gas pressure increases and lifts the lid.

6. The equation to the right illustrates what pressure measures and the units we use to describe it. The units of pressure are used throughout this book in describing weather, climate, and the flow of water.
7. Pressure is a force exerted on a given surface area.
8. According to Newton’s second law, force is the product of mass and acceleration.
9. The unit of mass is the kilogram (kg), acceleration is in meters/second/ second (m/s2), and area is in square meters (m2). 10. A force of one kg m/s2 is called a Newton. Pressure, measured in Pascals, is an expression of the number of Newtons of force exerted on a square meter of surface.
11. The air pressure at Earth’s surface is many Pascals, so we express pressures in a larger, related unit called a bar, or in millibars (1/1000 of a bar).
How Is Air Pressure Measured?
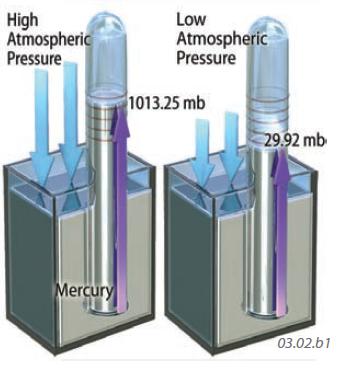
We can measure air pressure with an instrument called a barometer. The barometer shown to the left is a sealed glass tube fixed in liquid mercury. Changes in air pressure cause the liquid level in the tube to rise or fall, allowing the measurement of relative pressure. Such barometers have units of inches (or centimeters) of mercury. Pressure is also reported in units of a bar, with one bar being approximately equal to the average air pressure at sea level. Modern digital instruments record pressure in millibars.
Meteorologists measure air pressure at vertical heights in the atmosphere using hydrogen- or helium-filled balloons, like this one. An instrument package called a radiosonde is suspended from the balloon (lower left of the photograph). Sensors measure pressure, temperature, humidity, and position (using a GPS) as the balloon ascends, and these measurements are transmitted via radio to a central computer. Wind speed and direction are inferred from successive positions of the radiosonde. The balloon eventually pops and the radiosonde parachutes to the ground.

How Does Air Pressure Vary Vertically?
1. Air pressure in the atmosphere is not constant. The largest variation is vertically, with an abrupt decrease in pressure upward from near the surface. The red curve on this figure shows how the air pressure, measured in millibars (mb), decreases from Earth’s surface to the top of the atmosphere.
2. This diagram depicts the main layers of the atmosphere (troposphere, stratosphere, etc.) and highlights some of the features observed in each part, such as auroras in the thermosphere, shooting stars that mostly burn up in the mesosphere, and the restriction of most clouds and weather to the troposphere.
3. Colors along the left edge of the diagram convey temperature variations within and between the atmospheric layers. These vertical temperature variations affect the density of the air, impacting air motions caused by the Sun heating the Earth’s surface.
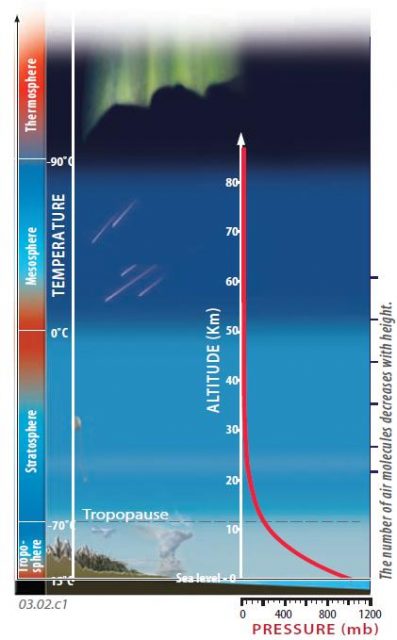
4. In the thermosphere and mesosphere, gas molecules are relatively sparse and temperatures are low (?90°C at the thermosphere mesosphere boundary). As a result of the sparseness of molecules, air pressures are very low (less than one millibar).
5. The abundance of gas molecules increases down into the stratosphere, and this is accompanied by an increase in air pressure (the bending of the red curve to the right as it goes downward). Air pressures are slightly greater at the top of the stratosphere than in the overlying mesosphere, but at the base of the stratosphere (the tropopause) they have increased to about one-fifth of pressures measured at sea level.
6. The pull of Earth’s gravity holds most gas molecules close to Earth’s surface, in the troposphere. Air pressure increases downward in the troposphere because of a greater abundance of molecules downward and the larger total number of molecules pressing down from the layers above. The highest air pressures are close to the surface, and at the lowest elevations. Sea level is the reference level for air pressure, with an average pressure of 1,013 mb (a little over 1 bar).
How Does Air Pressure Vary Laterally?
1. Air pressure also varies laterally, from area to area, and from hour to hour, and these variations are typically represented on maps, like the one shown here. Such maps either show the pressure conditions at a specific date and time or show pressure values averaged over some time period, like a month or a year. To allow us to compare different regions and to see the larger patterns, the map uses pressure values that are corrected to sea level, or their sea-level equivalent. In this way, we eliminate the effects of differences in elevation from place to place.
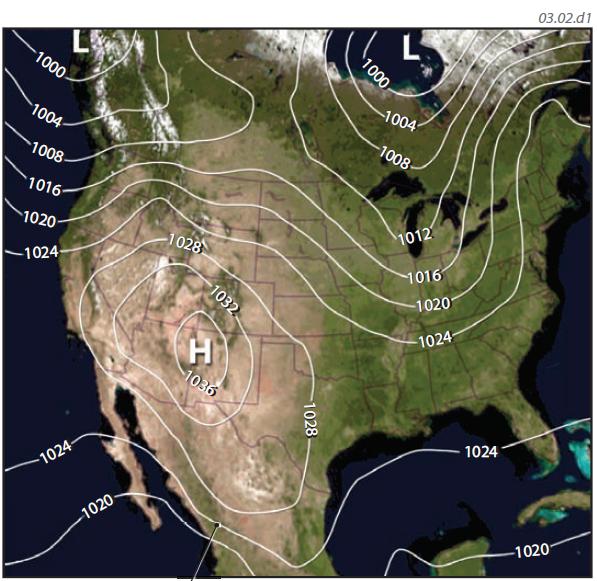
2. Such maps of air pressure contain numbered lines, called isobars, that connect locations with equal pressure. If you could follow an isobar across the countryside, you would follow a path along which the pressure values, once corrected to their sea-level equivalents, would be equal. Successive isobars are numbered to represent different values of air pressure, usually in millibars (e.g., 1,020), and there is generally a constant difference in pressure between two adjacent isobars (a 4 mb difference on this map). Note that isobars do not cross, but can completely encircle an area.
3. Most maps of air pressure feature the large capital letters H and L. An H represents an area of relatively higher pressure called a high-pressure area or simply a high. An L represents a low-pressure area, commonly called a low. An elongated area of high pressure can be called a ridge of high pressure and an elongated area of low pressure is a trough.
4. The map patterns change with time, corresponding to changes in air pressure that accompany changes in weather. Patterns typical for a region also change from season to season.
- How Do Gases Respond to Changes in Temperature and Pressure?
- How Do We Evaluate Sites for Solar-Energy Generation?
- How Are Variations in Insolation Expressed Between the North and South Poles?