How Do Gases Respond to Changes in Temperature and Pressure?
THE ATMOSPHERE CONSISTS LARGELY OF GASES, with lesser amounts of liquids, such as drops of water, and solids, such as dust and ice. By nature, gases expand easily or contract in volume in response to changes in temperature and pressure. Variations in temperature and resulting changes in pressure are the main drivers of motion in the atmosphere.
How Does a Gas Behave When Heated or Cooled?
The quantity of insolation entering the atmosphere exhibits considerable spatial variability, especially as a function of latitude, and temporal variations on both daily and seasonal time scales. These variations in insolation in turn lead to differences in temperatures. How do the gases in the atmosphere respond to changes in temperature?
1. Consider what happens when we want to make a hot-air balloon rise. Typically, a propane-powered burner heats ambient air, causing the air to expand in volume. This increase in volume inflates the balloon. Since the same amount of gas now occupies a much larger volume, the density of the heated air is less than the density of the surrounding air, so the balloon rises. So, as air increases in temperature, it tends to increase in volume and become less dense.

2. The figure below shows how a quantity of gas responds to either an increase in temperature (heating) or a decrease in temperature (cooling). The starting condition is represented by the cube of gas on the left.
3. An increase in the temperature of a gas means more energetic molecules, so a larger volume is needed to accommodate the same amount of gas.
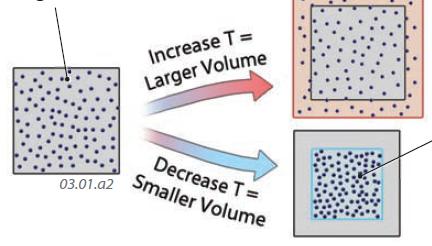
4. If a gas cools, the molecules within it have less kinetic energy (motions) and can therefore be packed into a smaller volume. The gas has a higher density and will tend to sink.
5. This example shows that temperature and volume of a gas are directly related — in fact they are proportional if pressure is held constant. Such a proportional relationship means that if temperature is doubled, volume doubles too. If temperature decreases by half, volume does too. This specific relationship is called Charles’s Law, which is one of the fundamental laws governing the behavior of gases, and it explains why a hot air balloon rises.
What Happens When a Gas Is Compressed?
If a gas is held at a constant temperature but forced to occupy a smaller volume, the pressure of the gas increases. Pressure is proportional to the number of collisions of the molecules. If the same gas fills a larger volume, the pressure decreases. In both cases, if we instead change the pressure, the volume of the gas will adjust accordingly. A material, like a gas, that can be compressed, is said to be compressible.
1. Molecules of gas in the sealed container in the left canister are under pressure, represented by the two weights resting on top. At some temperature, the molecules have a fixed amount of energy, and some of the moving molecules are hitting the moveable lid, resisting the downward force of the attached weight.
2. Removing a weight reduces the downward pressure on the gas. However, the gas retains its same average energy level (temperature) and therefore exerts the same upward force on the moveable lid as before. The upward force from the gas molecules exceeds the downward force of the weight and so raises the lid, increasing the volume occupied by the gas. In this way, a decrease in pressure results in an increase in volume, if the gas does not change temperature.
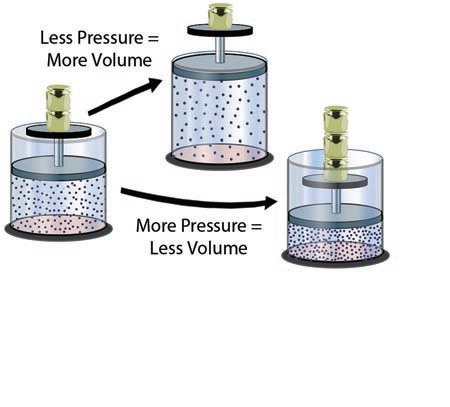
3. Increasing the downward pressure by adding weight on the original canister causes the lid to slide down. This increase in pressure causes a decrease in volume. As the gas is compressed into a smaller volume, the number of the molecules impacting the lid increases. When this upward force from the gas molecules equals the downward force from the weight, the lid stops moving, and the volume and pressure of the gas stop changing.
4. The relationship between pressure and volume of a gas, under conditions of constant temperature, is inversely proportional — if pressure increases, volume decreases. If pressure decreases, volume increases. Either pressure or volume can change, and the other factor responds accordingly, changing in the opposite direction by a proportional amount. That is, if the volume is cut in half, the pressure doubles. If the volume doubles, the pressure is cut in half. This inversely proportional relationship between pressure and volume, under constant temperature, is called Boyle’s Law.
How Are Temperatures and Pressures Related?
Since Charles’s Law relates volume to temperature, and Boyle’s Law relates volume to pressure, we might suspect that we can relate temperature and pressure. Combining Charles’s Law and Boyle’s Law leads to the Ideal Gas Law, which relates temperature, pressure, and density (mass divided by volume). Basic aspects of the Ideal Gas Law help explain the processes that drive the motion of matter and associated energy in the atmosphere.
1. We can represent the Ideal Gas Law with a figure, with words, or with an equation. We begin with this figure, which expresses the two sides of the equation. On one side of the equation (the left in this figure) is pressure. On the right side of the equation are density and temperature. What the Ideal Gas Law states is that if we increase a variable on one side of the equation (like increasing pressure), then one or both of the variables on the other side of the equation have to change in the same direction — density or temperature have to also change, or perhaps both do.
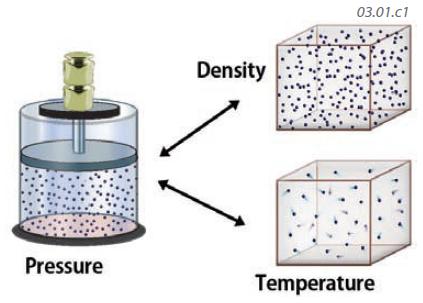
2. Examine this figure and envision changing any one of the three variables (pressure, density, and temperature), and consider how the other two variables would respond to satisfy the visual equation.
3. What happens if pressure increases? If temperature does not change, then density must increase. If pressure increases but density does not change, then temperature has to increase. Alternatively, temperature and density can both change. This three-way relationship partly explains why temperatures are generally warmer and the air is more dense at low elevations, where the air is compressed by the entire weight of the atmosphere, than at higher elevations, where there is less air. Higher pressure often results in higher temperatures.
4. What does the relationship predict will happen if a gas is heated to a higher temperature? If the density does not change, the pressure exerted by the gas on the plunger must increase. If the pressure does not change, the density must decrease. This is because density and temperature are on the same side of the equation, so an increase in one must be matched by a decrease in the other — if the other side of the equation (pressure) does not change. The relationship indicates that heated air can become less dense, which allows it to rise, like in a hot air balloon.
5. The Ideal Gas Law can also be expressed by the equation to the right: P = R p T where P is pressure, R is a constant,p is density (shown by the greek letter rho), and T is temperature. Note how this equation roughly corresponds to the figure above.
How Can Differences in Insolation Change Temperatures,
Pressures, and Density in the Atmosphere?
The way gas responds to changes in temperature and pressure is the fundamental driver of motion in the atmosphere. Since temperature changes are largely due to insolation, we can examine how insolation affects the physical properties of gas and how this drives atmospheric motion.
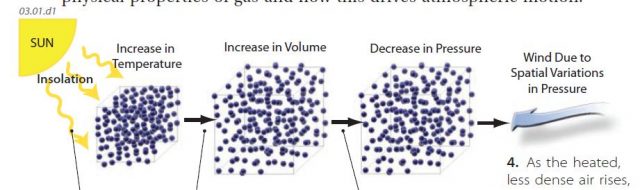
1. The Sun is the major energy source for Earth’s weather, climate, and movements of energy and matter in the atmosphere and oceans. In the figure above, insolation strikes Earth’s surface (land or water), which in turn heats a volume of gas in the overlying atmosphere.
2. The increase in temperature results in expansion of the gas because of the increased kinetic energy of the molecules in the gas, which is an increase in volume. If the same number of gas molecules occupy more volume, the density of the air decreases (the air becomes less dense).
3. The increase in volume can result in a decrease in pressure (less frequent molecular collisions). As a result, the air mass is now less dense than adjacent air that was heated less. The more strongly heated and expanded air rises because it is less dense relative to surrounding air (which was not heated as much and so is more dense).
4. As the heated, less dense air rises, adjacent air flows into the area to replace the rising air. The end result is a vertical and lateral movement of air within the atmosphere — vertical motion within and above the rising air, and lateral motion of surrounding air toward the area vacated by the rising air.
5. In this way, the response of gas to changes in temperature, pressure, and density (or volume), as expressed by the gas laws, is the primary cause of motion in the atmosphere. Variations in insolation cause changes in temperature, pressure, and density, which in turn cause air to move within the atmosphere.