What Is Humidity?
THE AMOUNT OF WATER VAPOR in the air is referred to as humidity. Humidity is something we can sense, affecting whether the air feels humid or dry. We are most familiar with one measure of humidity — relative humidity, a term commonly used on daily weather reports. There are other measures of humidity, some of which are more useful for comparing the amount of moisture between different elevations, times, and regions. Understanding humidity leads to a much better understanding of weather and climate.
What Are Humidity and Vapor Pressure?
The atmosphere is nearly all nitrogen and oxygen, but it contains a small but variable (<1–4%) amount of water vapor (and other gases). The term humidity conveys the amount of water vapor in the atmosphere, and the amount of humidity can be represented in several ways.
Humidity and Vapor Pressure
1. Imagine two cubes, both the same size and partially filled with water but mostly filled with air. In both cubes, some water molecules have evaporated from the liquid, becoming water vapor in the air. The amount of water vapor in the air is the humidity of the air. In this figure, water vapor is represented by small blue dots in the air.
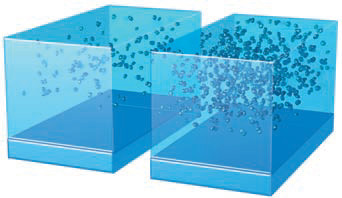
2. In the example shown here, the cube on the right has more water vapor than does the left cube, so the air in the right cube has a higher humidity than does air in the left cube. If we captured and weighed all the water vapor in either cube, we could determine how much water vapor was in that volume of air. Such a measure, called absolute humidity, is in units of mass per volume, such as grams per cubic meter (g/m3). Since this value changes as air expands or compresses (changes in volume), as commonly occurs in the atmosphere, absolute humidity is not a very useful measure in understanding the atmosphere.
3. Recall that the weight of the atmosphere pushing down causes atmospheric pressure. Some amount of this pressure is due to the weight of the water vapor that is part of the atmosphere, and this provides us with another way to represent humidity. The amount of pressure contributed by the water vapor is, not surprisingly, called the vapor pressure. Vapor pressure is expressed using the same units as atmospheric pressure, such as millibars (mb). For a given volume of air, if there are more water vapor molecules present, then the vapor pressure is higher. In meteorology, vapor pressure is represented by the small letter e.
Water-Vapor Capacity
4. Air can include only a limited amount of water vapor, and the maximum amount it can include is called its water-vapor capacity (commonly depicted by es, where the s stands for saturation). The water-vapor capacity of air varies by the temperature of that air, as shown in the graph below. The blue curve represents the maximum amount of water vapor at different temperatures, with the water vapor capacity increasing exponentially with increasing temperature — warmer air has a greater capacity for water vapor than does cooler air. Water-vapor capacity can be expressed in millibars (mb).
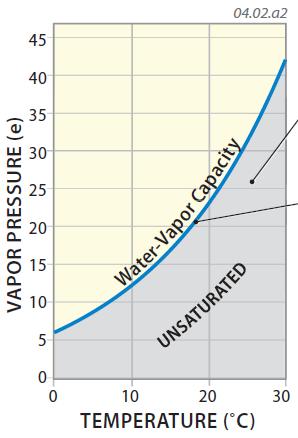
5. If the conditions of temperature and vapor pressure for some volume of air plot in the shaded area on this graph, even more water vapor can exist in the air and the air is unsaturated. These are the conditions we normally experience when the weather is fair (not raining or snowing).
6. If the conditions plot directly on the blue line, the air has as much water vapor as possible — it is saturated with respect to water vapor. Under these conditions, the water vapor begins to form droplets via the process of condensation or ice crystals via the process of deposition. In other words, when the atmosphere reaches saturation, drops of water form, such as in clouds and fog, perhaps followed by precipitation. In certain settings, conditions can be above the blue line, and the air is said to be supersaturated.
Relative Humidity
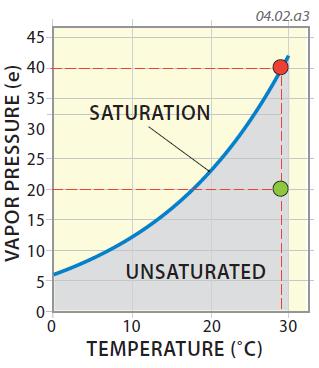
7. A common way to convey humidity is to express it as a ratio of how much water vapor is in the air relative to the maximum amount of water vapor that is possible (i.e., the water-vapor capacity). In the graph below, the green dot represents a vapor pressure of 20 mb and a temperature of 29°C. At that temperature, saturation would occur at about 40 mb, so the vapor pressure (humidity) represented by the green dot is half, or 50% of the maximum possible. The atmosphere is unsaturated in this case. This percentage, representing the observed vapor pressure divided by the maximum possible vapor pressure (i.e., water-vapor capacity), is the relative humidity, the term we hear on daily weather reports. Relative humidity varies from single digits (e.g., 5%) in very dry places, such as dry seasons in a desert, to 90% to 100% in very humid conditions.
What Is Specific Humidity?
Relative humidity helps describe how humid the air feels to us and indicates how close the air is to saturation, but relative humidity varies with temperature, even if the amount of water vapor in the air has not changed. Therefore, we also use another measurement of humidity, called specific humidity, which compares the mass of water vapor in a body of air to the total mass of that air. An advantage of this approach is that a specific-humidity measurement is not affected by changes in temperature, pressure, and volume that occur when air masses move, such as up or down in elevation. Specific humidity, therefore, is a convenient way of comparing the amount of moisture at different elevations, at different times, and from region to region.
1. Specific humidity is expressed as the ratio of the mass of water vapor in some body of air to the total mass of that air. Specific humidity is represented by a lowercase q and is calculated in the example below.
2. This cube contains molecules of water vapor (shown as blue dots) dispersed through a mass of atmosphere (filling the rest of the cube). In this example, the mass of all the water vapor is 12 grams (g), and the total mass of the air (including the water vapor) is 1 kilogram (kg). The specific humidity is therefore the following ratio:
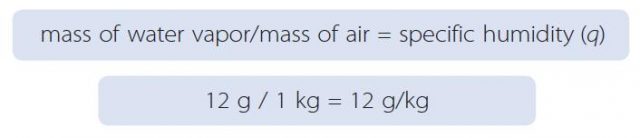
3. The calculated specific humidity of 12 g/kg is a typical value for the air. We use the units of g/kg because the amount of water vapor is small compared to the total amount of air.
4. Note that since specific humidity is calculated with the masses of water vapor and air, it is not directly dependent on the temperature, even though places with different temperatures often have very different specific humidities. It is also largely independent of changes in volume (and therefore pressure) — even if we shrink the cube it will still contain the same ratio of the mass of water vapor to total mass of air. The specific humidity of an air mass therefore changes only slightly as that air warms, cools, or changes pressure as it moves in the atmosphere. The specific humidity can only be changed by adding or subtracting water vapor, such as adding water vapor through evaporation of surface waters or by losing water vapor through the formation of precipitation.
Specific Humidity and Water-Vapor Capacity
5. This graph plots specific humidity and temperature, with the blue line showing conditions where air is saturated with water vapor. The curve has the same shape as the curves on the other page because it also represents water-vapor capacity. It curves strongly upward with higher temperatures. The curve does not show how specific humidity of an air mass varies with temperature, only the conditions under which it becomes saturated. The shaded field represents conditions where air is unsaturated (can evaporate even more water vapor).
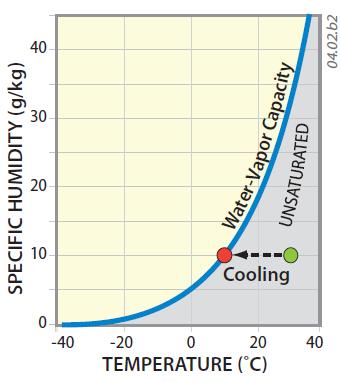
6. At low temperatures, represented by the left part of the graph, much less water vapor can exist than when air is warmer (the right part of the graph).
7. The green dot represents air that has a specific humidity of 10 g/kg and a temperature of 30°C. If we cool the air (moving left on the graph), the air can reach saturation (the red dot), but note that the specific humidity does not change, because it is a ratio of masses. Seen in this way, the air's ability to evaporate moisture does change with temperature (the blue curve), but specific humidity tells us the concentration of water vapor in the air, independent of temperature. For this reason, specific humidity is very useful for comparing the moisture content of air in different settings.
Comparing Different Measures of Humidity
8. The table below lists water-vapor capacity, specific humidity, and relative humidity for air at four different temperatures. Note that the specific humidity does not change as the air cools, but the relative humidity increases until it reaches 100% at 10°C (i.e., the air becomes saturated).