What Is the Atmosphere?
A RELATIVELY THIN LAYER OF GAS — the atmosphere — surrounds Earth's surface. The atmosphere shields us from harmful high-energy rays from space, is the source of our weather and climate, and contains the oxygen, water vapor, and other gases on which all life depends. What is the character and composition of the atmosphere, and how does it interact with light coming from the Sun?
What Is the Character of the Atmosphere?
Examine this view of Earth, taken from a spacecraft orbiting high above the Earth. As viewed from space, Earth is dominated by three things: the blue oceans and seas, the multicolored land, and clouds. If you look closely at the very edge of the planet, you can observe a thin blue fringe that is the atmosphere. From this perspective, the atmosphere appears to be an incredibly thin layer that envelopes our planet, separating us from the dark vastness of space. Clouds, which are so conspicuous in any image of Earth taken from space, mostly circulate within the lower atmosphere, bringing rain and snow. The winds that move the clouds are also within the atmosphere.

As viewed from the ground, the atmosphere mostly appears as a blue sky with variable amounts of clouds, which are commonly nearly white or some shade of gray. During sunset and sunrise, the sky can glow reddish or orange. The colors of the sky and clouds are due to the way sunlight interacts with matter in the atmosphere.

What Is the Structure of the Atmosphere?
The atmosphere extends from the surface of Earth upward for more than 100 km, with some characteristics of the atmosphere going out to thousands of kilometers. The atmosphere is not homogeneous in any of its attributes, but instead has different layers that vary in temperature, air pressure, and the amount and composition of gases. Each layer has the term “sphere” as part of its name, referring to the way each layer successively wraps around the Earth with a roughly spherical shape. Examine the figure below and then read the text from the bottom left, starting with the lowest and most familiar part of the atmosphere.
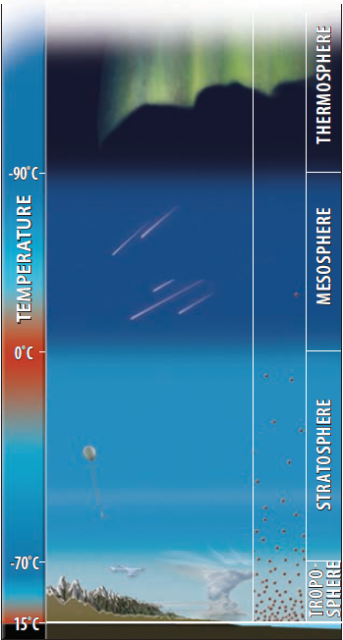
On the right side of this figure are scattered bright dots that represent gas molecules in the atmosphere. The molecules are infinitely smaller and more abundant than shown here. Note that the molecules are more abundant lower in the atmosphere and become much less abundant upward. Over 70% of the mass of the atmosphere is in the lowest 10 km, that is, within the troposphere. The mesosphere and thermosphere contain only a few tenths of a percent of the atmosphere's mass.
The top layer of the atmosphere is the thermosphere, derived from the Greek word for heat because this layer, surprisingly, can become very hot (more than 1,500°C) as gas particles intercept the Sun's energy. It is the altitude where the spectacular auroras (e.g., “Northern Lights”) originate from interactions of solar energy and energetic gas molecules.
Above the stratosphere is the mesosphere, where “meso” is Greek for “middle,” in reference to this layer being in the middle of the atmosphere. The mesosphere starts at 50 km, the top of the stratosphere, and goes up to more than 80 km (~50 miles) in altitude. The upper part of the mesosphere is very cold (?85°C, ?120°F), and is considered by many scientists to be the coldest place within the Earth system. It is within the mesosphere that most small meteors burn up, producing the effect called “shooting stars.” Radio waves from Earth bounce off this layer and the thermosphere, allowing us to hear radio stations from far away.
The next layer up is the stratosphere, beginning at an altitude of about 10 km above sea level, at about the elevation of Earth's highest peaks. The name is derived from a Latin term for spreading out, referring to its layered (not mixed) character. The name also helps convey that temperatures are stratified, varying from cooler lower altitudes to warmer upper ones. The lowest part of the stratosphere is an altitude at which many commercial jets fly because the air offers less resistance to motion, allowing appreciable fuel savings.
The lowest layer of the atmosphere is the one with which we surface-dwellers interact. It contains the air we breathe, clouds, wind, rain, and other aspects of weather. This layer is the troposphere, with the name “tropo” being derived from a Greek word for turning or mixing, in reference to the swirling motion of clouds, wind, and other manifestations of weather.
What Is the Composition of the Atmosphere?
The atmosphere is not completely homogeneous in its vertical, horizontal, or temporal composition, but chemists and atmospheric scientists have estimated its average composition, as represented in the graph and table below.
The atmosphere is held in place by the balance between gravity (which is directed downward and keeps the gases close to the surface) and a buoyancy force (which is directed upward and exists because material tends to flow toward the vacuum of outer space). Near the surface, the greater weight of the overlying atmosphere results in more molecules being tightly packed close to the surface of the Earth and a rapid thinning of the number of molecules with distance upward in the atmosphere, away from the surface. This effect of gravity also influences the composition of the atmosphere, with a higher proportion of heavier gases low in the atmosphere (in the troposphere) and a higher proportion of lighter gases, such as hydrogen, higher up in the atmosphere.
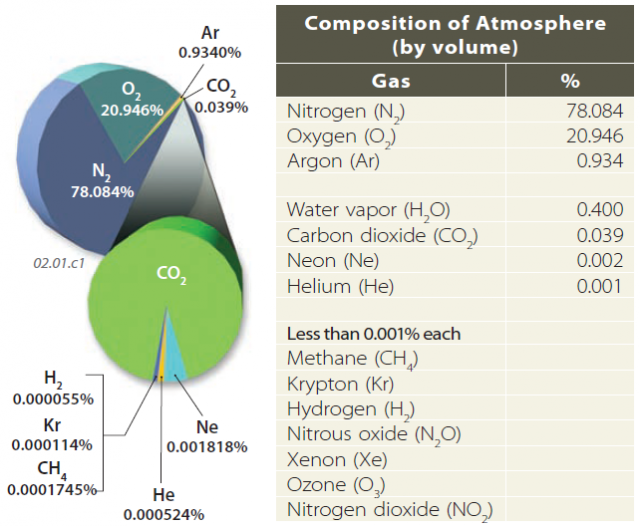
As shown by this diagram and table, the two dominant gases are nitrogen (78%) and oxygen (21%), followed by argon. Several other gases, such as water vapor, carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and ozone (O3) play significant roles in global climate through their interaction with energy emitted by the Sun and re-emitted by the Earth.
The atmosphere also contains various types of solids and liquids called aerosols, such as dust, industrial pollutants, and tiny drops of liquid from volcanic eruptions. Aerosols play an important role in the energy balance of the Earth and aid in the formation of clouds and precipitation.
How Does the Atmosphere Interact with Energy from the Sun?
Gas in the atmosphere interacts with visible light and other energy radiated by the Sun, as well as energy reflected from and radiated from Earth's surface. An understanding of the possible types of interactions helps explain what we see every day, such as colors, as well as the underlying causes of weather and climate. Here we discuss four types of interactions: transmission, reflection, absorption, and scattering. The discussion will emphasize light, but the principles are applicable to other forms of radiant energy, such as ultraviolet energy.
Transmission — An object can be entirely or mostly transparent to light and other forms of radiant energy, in the way that a clear glass sphere permits most light to pass through. Allowing such energy to pass through is called transmission. The atmosphere is largely transparent to visible light, allowing it to pass through and illuminate Earth's surface. For some types of radiant energy the atmosphere is only partially transparent, because certain molecules interact with some of the incoming radiation.
Energy from the Sun is efficiently transmitted through space, which is nearly a vacuum, lacking many molecules that could interact with the energy. Reflection — Instead of passing through an object, some light can bounce off the object, the process of reflection, as illustrated by light reflecting off a polished metal sphere. Not all light and energy bounce off real objects, so reflectivity can be thought of as a continuum from objects being perfectly reflective to having no reflectivity. For visible light, objects that are white or made of shiny, polished metal are highly reflective, whereas dark, rough objects have low reflectivities. In the summer sun, a white car, which reflects much of the Sun's heat, is cooler than a dark car that is not so reflective. If an object, like a leaf, preferentially reflects green light, we see the object as green.
Absorption — Objects retain some of the energy that strikes them, and this process of retention is called absorption. Objects have varying degrees of absorption. This dark, dull-textured sphere is highly absorbent, retaining most of the light that strikes it, accounting for the sphere's dark color (not much light coming back off the surface). Energy that is absorbed by an object can be released back in another form (like heat from the dark ball). The giving out of radiant energy is called emission.
Scattering — Most objects reflect some light, but in a way that disperses the energy in various directions, scattering the light. The shiny but rough sphere shown here has a highly reflective surface, but the roughness causes light to be scattered in various directions. Such scattering occurs when light and other energy strike the land, and it is also caused by certain types of aerosols and gases, like water vapor, in the atmosphere. Gases in the atmosphere preferentially scatter blue light, which spreads out in all directions through the atmosphere, causing the dominantly blue color of the sky.